The Inflammatory response induced by aspartic proteases of Candida albicans is independent of proteolytic activity
- PMID: 20713630
- PMCID: PMC2976325
- DOI: 10.1128/IAI.00789-10
The Inflammatory response induced by aspartic proteases of Candida albicans is independent of proteolytic activity
Abstract
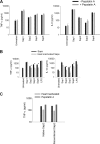
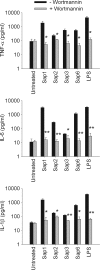
The secretion of aspartic proteases (Saps) has long been recognized as a virulence-associated trait of the pathogenic yeast Candida albicans. In this study, we report that different recombinant Saps, including Sap1, Sap2, Sap3, and Sap6, have differing abilities to induce secretion of proinflammatory cytokines by human monocytes. In particular Sap1, Sap2, and Sap6 significantly induced interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), and IL-6 production. Sap3 was able to stimulate the secretion of IL-1β and TNF-α. All Saps tested were able to induce Ca(2+) influx in monocytes. Treatment of these Saps with pepstatin A did not have any effect on cytokine secretion, indicating that their stimulatory potential was independent from their proteolytic activity. The capacity of Saps to induce inflammatory cytokine production was also independent from protease-activated receptor (PAR) activation and from the optimal pH for individual Sap activity. The interaction of Saps with monocytes induced Akt activation and phosphorylation of IκBα, which mediates translocation of NF-κB into the nucleus. Overall, these results suggest that individual Sap proteins can induce an inflammatory response and that this phenomenon is independent from the pH of a specific host niche and from Sap enzymatic activity. The inflammatory response is partially dependent on Sap denaturation and is triggered by the Akt/NF-κB activation pathway. Our data suggest a novel, activity-independent aspect of Saps during interactions of C. albicans with the host.
Figures



References
-
- Albrecht, A., A. Felk, I. Pichova, J. R. Naglik, M. Schaller, P. de Groot, D. Maccallum, F. C. Odds, W. Schafer, F. Klis, M. Monod, and B. Hube. 2006. Glycosylphosphatidylinositol-anchored proteases of Candida albicans target proteins necessary for both cellular processes and host-pathogen interactions. J. Biol. Chem. 281:688-694. - PubMed
-
- Borelli, C., E. Ruge, J. H. Lee, M. Schaller, A. Vogelsang, M. Monod, H. C. Korting, R. Huber, and K. Maskos. 2008. X-ray structures of Sap1 and Sap5: structural comparison of the secreted aspartic proteinases from Candida albicans. Proteins 72:1308-1319. - PubMed
-
- Borg-von Zepelin, M., S. Beggah, K. Boggian, D. Sanglard, and M. Monod. 1998. The expression of the secreted aspartyl proteinases Sap4 to Sap6 from Candida albicans in murine macrophages. Mol. Microbiol. 28:543-554. - PubMed
-
- Brunke, S., and B. Hube. 2006. MfLIP1, a gene encoding an extracellular lipase of the lipid-dependent fungus Malassezia furfur. Microbiology 152:547-554. - PubMed
-
- Calderone, R. A., and W. A. Fonzi. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327-335. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

