Sulfated derivatives of Escherichia coli K5 capsular polysaccharide are potent inhibitors of human cytomegalovirus
- PMID: 20713657
- PMCID: PMC2976136
- DOI: 10.1128/AAC.00721-10
Sulfated derivatives of Escherichia coli K5 capsular polysaccharide are potent inhibitors of human cytomegalovirus
Abstract
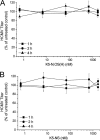
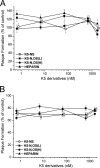
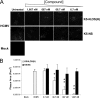
To date, there are few drugs licensed for the treatment of human cytomegalovirus (HCMV) infections, most of which target the viral DNA polymerase and suffer from many drawbacks. Thus, there is still a strong need for new anti-HCMV compounds with novel mechanisms of action. In this study, we investigated the anti-HCMV activity of chemically sulfated derivatives of Escherichia coli K5 capsular polysaccharide. These compounds are structurally related to cellular heparan sulfate and have been previously shown to be effective against some enveloped and nonenveloped viruses. We demonstrated that two derivatives, i.e., K5-N,OS(H) and K5-N,OS(L), are able to prevent cell infection by different strains of HCMV at concentrations in the nanomolar range while having no significant cytotoxicity. Studies performed to elucidate the mechanism of action of their anti-HCMV activity revealed that these compounds do not interact with either the host cell or the viral particle but need a virus-cell interaction to exert antiviral effects. Furthermore, these K5 derivatives were able to inhibit the attachment step of HCMV infection, as well as the viral cell-to-cell spread. Since the mode of inhibition of these compounds appears to differ from that of the available anti-HCMV drugs, sulfated K5 derivatives could represent the basis for the development of a novel class of potent anti-HCMV compounds. Interestingly, our studies highlight that small variations of the K5 derivatives structure can modulate the selectivity and potency of their activities against different viruses, including viruses belonging to the same family.
Figures
Similar articles
-
Highly sulfated K5 Escherichia coli polysaccharide derivatives inhibit respiratory syncytial virus infectivity in cell lines and human tracheal-bronchial histocultures.Antimicrob Agents Chemother. 2014 Aug;58(8):4782-94. doi: 10.1128/AAC.02594-14. Epub 2014 Jun 9. Antimicrob Agents Chemother. 2014. PMID: 24914125 Free PMC article.
-
Sulfated K5 Escherichia coli polysaccharide derivatives as wide-range inhibitors of genital types of human papillomavirus.Antimicrob Agents Chemother. 2008 Apr;52(4):1374-81. doi: 10.1128/AAC.01467-07. Epub 2008 Feb 4. Antimicrob Agents Chemother. 2008. PMID: 18250186 Free PMC article.
-
Sulfated K5 Escherichia coli polysaccharide derivatives: A novel class of candidate antiviral microbicides.Pharmacol Ther. 2009 Sep;123(3):310-22. doi: 10.1016/j.pharmthera.2009.05.001. Epub 2009 May 14. Pharmacol Ther. 2009. PMID: 19447134 Review.
-
Inhibition of herpes simplex virus types 1 and 2 in vitro infection by sulfated derivatives of Escherichia coli K5 polysaccharide.Antimicrob Agents Chemother. 2008 Sep;52(9):3078-84. doi: 10.1128/AAC.00359-08. Epub 2008 Jun 23. Antimicrob Agents Chemother. 2008. PMID: 18573926 Free PMC article.
-
Early inhibitors of human cytomegalovirus: state-of-art and therapeutic perspectives.Pharmacol Ther. 2011 Sep;131(3):309-29. doi: 10.1016/j.pharmthera.2011.04.007. Epub 2011 Apr 28. Pharmacol Ther. 2011. PMID: 21570424 Free PMC article. Review.
Cited by
-
Antiviral activities of four marine sulfated glycans against adenovirus and human cytomegalovirus.Antiviral Res. 2021 Jun;190:105077. doi: 10.1016/j.antiviral.2021.105077. Epub 2021 Apr 14. Antiviral Res. 2021. PMID: 33864843 Free PMC article.
-
New pharmacological strategies to fight enveloped viruses.Trends Pharmacol Sci. 2014 Sep;35(9):470-8. doi: 10.1016/j.tips.2014.06.004. Epub 2014 Aug 6. Trends Pharmacol Sci. 2014. PMID: 25108320 Free PMC article. Review.
-
Pathogenesis and Inhibition of Flaviviruses from a Carbohydrate Perspective.Pharmaceuticals (Basel). 2017 May 4;10(2):44. doi: 10.3390/ph10020044. Pharmaceuticals (Basel). 2017. PMID: 28471403 Free PMC article. Review.
-
Highly sulfated K5 Escherichia coli polysaccharide derivatives inhibit respiratory syncytial virus infectivity in cell lines and human tracheal-bronchial histocultures.Antimicrob Agents Chemother. 2014 Aug;58(8):4782-94. doi: 10.1128/AAC.02594-14. Epub 2014 Jun 9. Antimicrob Agents Chemother. 2014. PMID: 24914125 Free PMC article.
-
Heparan Sulfate Proteoglycans and Viral Attachment: True Receptors or Adaptation Bias?Viruses. 2019 Jul 1;11(7):596. doi: 10.3390/v11070596. Viruses. 2019. PMID: 31266258 Free PMC article. Review.
References
-
- Britt, W. 2008. Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr. Top. Microbiol. Immunol. 325:417-470. - PubMed
-
- Casu, B., G. Diamantini, G. Fedeli, M. Mantovani, P. Oreste, R. Pescador, R. Porta, G. Prino, G. Torri, and G. Zoppetti. 1986. Retention of antilipemic activity by periodate-oxidized non-anticoagulant heparins. Arzneimittelforschung 36:637-642. - PubMed
-
- Casu, B., and U. Gennaro. 1975. A conductimetric method for the determination of sulphate and carboxyl groups in heparin and other mucopolysaccharides. Carbohydr. Res. 39:168-176. - PubMed
-
- Casu, B., G. Grazioli, N. Razi, M. Guerrini, A. Naggi, G. Torri, P. Oreste, F. Tursi, G. Zoppetti, and U. Lindahl. 1994. Heparin-like compounds prepared by chemical modification of capsular polysaccharide from E. coli K5. Carbohydr. Res. 263:271-284. - PubMed
-
- Compton, T. 2004. Receptors and immune sensors: the complex entry path of human cytomegalovirus. Trends Cell. Biol. 14:5-8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

