1-O-hexadecyloxypropyl cidofovir (CMX001) effectively inhibits polyomavirus BK replication in primary human renal tubular epithelial cells
- PMID: 20713664
- PMCID: PMC2976161
- DOI: 10.1128/AAC.00974-10
1-O-hexadecyloxypropyl cidofovir (CMX001) effectively inhibits polyomavirus BK replication in primary human renal tubular epithelial cells
Abstract
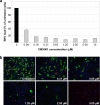
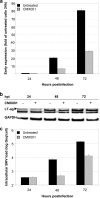
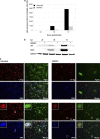
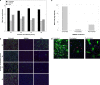
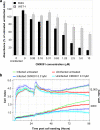
Antiviral drugs for treating polyomavirus BK (BKV) replication in polyomavirus-associated nephropathy or hemorrhagic cystitis are of considerable clinical interest. Unlike cidofovir, the lipid conjugate 1-O-hexadecyloxypropyl cidofovir (CMX001) is orally available and has not caused detectable nephrotoxicity in rodent models or human studies to date. Primary human renal proximal tubular epithelial cells were infected with BKV-Dunlop, and CMX001 was added 2 h postinfection (hpi). The intracellular and extracellular BKV DNA load was determined by quantitative PCR. Viral gene expression was examined by quantitative reverse transcription-PCR, Western blotting, and immunofluorescence microscopy. We also examined host cell viability, proliferation, metabolic activity, and DNA replication. The titration of CMX001 identified 0.31 μM as the 90% effective concentration (EC(90)) for reducing the extracellular BKV load at 72 hpi. BKV large T antigen mRNA and protein expression was unaffected at 24 hpi, but the intracellular BKV genome was reduced by 90% at 48 hpi. Late gene expression was reduced by 70 and 90% at 48 and 72 hpi, respectively. Comparisons of CMX001 and cidofovir EC(90)s from 24 to 96 hpi demonstrated that CMX001 had a more rapid and enduring effect on BKV DNA and infectious progeny at 96 hpi than cidofovir. CMX001 at 0.31 μM had little effect on overall cell metabolism but reduced bromodeoxyuridine incorporation and host cell proliferation by 20 to 30%, while BKV infection increased cell proliferation in both rapidly dividing and near-confluent cultures. We conclude that CMX001 inhibits BKV replication with a longer-lasting effect than cidofovir at 400× lower levels, with fewer side effects on relevant host cells in vitro.
Figures
References
-
- Bernhoff, E., T. J. Gutteberg, K. Sandvik, H. H. Hirsch, and C. H. Rinaldo. 2008. Cidofovir inhibits polyomavirus BK replication in human renal tubular cells downstream of viral early gene expression. Am. J. Transplant. 8:1413-1422. - PubMed
-
- Binet, I., V. Nickeleit, H. H. Hirsch, O. Prince, P. Dalquen, F. Gudat, M. J. Mihatsch, and G. Thiel. 1999. Polyomavirus disease under new immunosuppressive drugs: a cause of renal graft dysfunction and graft loss. Transplantation 67:918-922. - PubMed
-
- Binggeli, S., A. Egli, S. Schaub, I. Binet, M. Mayr, J. Steiger, and H. H. Hirsch. 2007. Polyomavirus BK-specific cellular immune response to VP1 and large T-antigen in kidney transplant recipients. Am. J. Transplant. 7:1131-1139. - PubMed
-
- Cole, C. N. 1996. Polyomavirinae: the viruses and their replication, p. 917-945. In D. M. K. Bernard, N. Fields, and Peter M. Howley (ed.), Fundamental virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, PA.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

