Efficacy and safety of intravenous peramivir for treatment of seasonal influenza virus infection
- PMID: 20713668
- PMCID: PMC2976170
- DOI: 10.1128/AAC.00474-10
Efficacy and safety of intravenous peramivir for treatment of seasonal influenza virus infection
Abstract
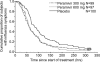
Peramivir, a sialic acid analogue, is a selective inhibitor of neuraminidases produced by influenza A and B viruses. We evaluated the efficacy and safety of a single intravenous dose of peramivir in outpatients with uncomplicated seasonal influenza virus infection. A total of 300 previously healthy adult subjects aged 20 to 64 years with a positive influenza virus rapid antigen test were recruited within 48 h of the onset of influenza symptoms and randomized to three groups: single intravenous infusion of either 300 mg peramivir per kg of body weight, 600 mg peramivir, or matching placebo on study day 1. Influenza symptoms and body temperature were self-assessed for 14 days. Nasal and pharyngeal swabs were collected to determine the viral titer. The primary endpoint was the time to alleviation of symptoms. Of the 300 subjects, 296 were included in the intent-to-treat infected population (300 mg peramivir, n = 99; 600 mg peramivir, n = 97; and placebo, n = 100). Peramivir significantly reduced the time to alleviation of symptoms at both 300 mg (hazard ratio, 0.681) and 600 mg (hazard ratio, 0.666) compared with placebo (adjusted P value, 0.0092 for both comparisons). No serious adverse events were reported. Peramivir was well tolerated, and its adverse-event profile was similar to that of placebo. A single intravenous dose of peramivir is effective and well tolerated in subjects with uncomplicated seasonal influenza virus infection.
Figures

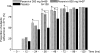

References
-
- Bantia, S., C. S. Arnold, C. D. Parker, R. Upshaw, and P. Chand. 2006. Anti-influenza virus activity of peramivir in mice with single intramuscular injection. Antiviral Res. 69:39-45. - PubMed
-
- Birnkrant, D., and E. Cox. 2009. The emergency use authorization of peramivir for treatment of 2009 H1N1 influenza. N. Engl. J. Med. 361:2204-2207. - PubMed
-
- Bright, R. A., M. J. Medina, X. Xu, G. Perez-Oronoz, T. R. Wallis, X. M. Davis, L. Povinelli, N. Cox, and A. I. Klimov. 2005. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. Lancet 366:1175-1181. - PubMed
-
- Deyde, V. M., X. Xu, R. A. Bright, M. Shaw, C. B. Smith, Y. Zhang, Y. Shu, L. V. Gubareva, N. J. Cox, and A. I. Klimov. 2007. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J. Infect. Dis. 196:249-257. - PubMed
-
- Division of Acquired Immunodeficiency Syndrome, NIAID, NIH. December 2004, posting date. Division of AIDS table for grading the severity of adult and pediatric adverse events. http://www3.niaid.nih.gov/research/resources/DAIDSClinRsrch/PDF/Safety/D....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

