miR-204 is required for lens and retinal development via Meis2 targeting
- PMID: 20713703
- PMCID: PMC2932609
- DOI: 10.1073/pnas.0914785107
miR-204 is required for lens and retinal development via Meis2 targeting
Abstract
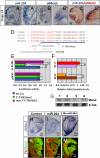
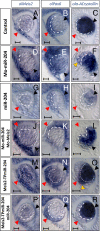
MicroRNAs (miRNAs) are small noncoding RNAs that have important roles in the regulation of gene expression. The roles of individual miRNAs in controlling vertebrate eye development remain, however, largely unexplored. Here, we show that a single miRNA, miR-204, regulates multiple aspects of eye development in the medaka fish (Oryzias latipes). Morpholino-mediated ablation of miR-204 expression resulted in an eye phenotype characterized by microphthalmia, abnormal lens formation, and altered dorsoventral (D-V) patterning of the retina, which is associated with optic fissure coloboma. Using a variety of in vivo and in vitro approaches, we identified the transcription factor Meis2 as one of the main targets of miR-204 function. We show that, together with altered regulation of the Pax6 pathway, the abnormally elevated levels of Meis2 resulting from miR-204 inactivation are largely responsible for the observed phenotype. These data provide an example of how a specific miRNA can regulate multiple events in eye formation; at the same time, they uncover an as yet unreported function of Meis2 in the specification of D-V patterning of the retina.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Meis homeoproteins directly regulate Pax6 during vertebrate lens morphogenesis.Genes Dev. 2002 Aug 15;16(16):2097-107. doi: 10.1101/gad.1007602. Genes Dev. 2002. PMID: 12183364 Free PMC article.
-
Growth and differentiation of the retina and the optic tectum in the medaka fish requires olSfrp5.Dev Neurobiol. 2009 Sep 1;69(10):617-32. doi: 10.1002/dneu.20731. Dev Neurobiol. 2009. PMID: 19507177
-
Genomic organization and embryonic expression of miR-430 in medaka (Oryzias latipes): insights into the post-transcriptional gene regulation in early development.Gene. 2010 Jan 1;449(1-2):41-9. doi: 10.1016/j.gene.2009.09.005. Epub 2009 Sep 19. Gene. 2010. PMID: 19770025
-
Eye organogenesis: A hierarchical view of ocular development.Curr Top Dev Biol. 2019;132:351-393. doi: 10.1016/bs.ctdb.2018.12.008. Epub 2019 Jan 3. Curr Top Dev Biol. 2019. PMID: 30797514 Review.
-
Zic Genes in Teleosts: Their Roles in Dorsoventral Patterning in the Somite.Adv Exp Med Biol. 2018;1046:141-156. doi: 10.1007/978-981-10-7311-3_8. Adv Exp Med Biol. 2018. PMID: 29442321 Review.
Cited by
-
MicroRNA-204 critically regulates carcinogenesis in malignant peripheral nerve sheath tumors.Neuro Oncol. 2012 Aug;14(8):1007-17. doi: 10.1093/neuonc/nos124. Epub 2012 Jun 19. Neuro Oncol. 2012. PMID: 22718995 Free PMC article.
-
Implication of the miR-184 and miR-204 competitive RNA network in control of mouse secondary cataract.Mol Med. 2012 May 9;18(1):528-38. doi: 10.2119/molmed.2011.00463. Mol Med. 2012. PMID: 22270329 Free PMC article.
-
MicroRNAs that target RGS5.Iran J Basic Med Sci. 2015 Feb;18(2):108-14. Iran J Basic Med Sci. 2015. PMID: 25810883 Free PMC article.
-
Downregulation of miR-204 expression defines a highly aggressive subset of Group 3/Group 4 medulloblastomas.Acta Neuropathol Commun. 2019 Apr 3;7(1):52. doi: 10.1186/s40478-019-0697-3. Acta Neuropathol Commun. 2019. PMID: 30944042 Free PMC article.
-
Plasmid-based target protectors allow specific blockade of miRNA silencing activity in mammalian developmental systems.Front Cell Neurosci. 2013 Sep 24;7:163. doi: 10.3389/fncel.2013.00163. eCollection 2013. Front Cell Neurosci. 2013. PMID: 24068984 Free PMC article.
References
-
- Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–296. - PubMed
-
- Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: Lessons from the retina. Nat Rev Neurosci. 2001;2:109–118. - PubMed
-
- Dyer MA, Cepko CL. Regulating proliferation during retinal development. Nat Rev Neurosci. 2001;2:333–342. - PubMed
-
- Schedl A, et al. Influence of PAX6 gene dosage on development: Overexpression causes severe eye abnormalities. Cell. 1996;86:71–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

