Uterine FK506-binding protein 52 (FKBP52)-peroxiredoxin-6 (PRDX6) signaling protects pregnancy from overt oxidative stress
- PMID: 20713718
- PMCID: PMC2932576
- DOI: 10.1073/pnas.1009324107
Uterine FK506-binding protein 52 (FKBP52)-peroxiredoxin-6 (PRDX6) signaling protects pregnancy from overt oxidative stress
Abstract
Immunophilin FK506-binding protein 52 (FKBP52) is a cochaperone that binds to the progesterone receptor (PR) to optimize progesterone (P(4))-PR signaling. We recently showed that Fkbp52-deficient (Fkbp52(-/-)) mice have reduced uterine PR responsiveness and implantation failure which is rescued by excess P(4) supplementation in a genetic background-dependent manner. This finding led us to hypothesize that FKBP52 has functions in addition to optimizing PR activity. Using proteomics analysis, we found that uterine levels of peroxiredoxin-6 (PRDX6), a unique antioxidant, are significantly lower in Fkbp52(-/-) mice than in WT and PR-null (Pgr(-/-)) mice. We also found that Fkbp52(-/-) mice with reduced uterine PRDX6 levels are susceptible to paraquat-induced oxidative stress (OS), leading to implantation failure even with P(4) supplementation. The same dose of paraquat did not interfere with implantation in WT mice. Moreover, treatment with antioxidants alpha-tocopherol and N-acetylcysteine (NAC) attenuated paraquat-induced implantation failure in P(4)-treated Fkbp52(-/-) mice. Functional analyses using mouse embryonic fibroblasts show that Fkbp52 deficiency associated with reduced PRDX6 levels promotes H(2)O(2)-induced cell death, which is reversed by the addition of NAC or by forced expression of PRDX6, suggesting that Fkbp52 deficiency diminishes the threshold against OS by reducing PRDX6 levels. These findings provide evidence that heightened uterine OS in Fkbp52(-/-) females with reduced PRDX6 levels induces implantation failure even in the presence of excess P(4). This study shows that FKBP52-PRDX6 signaling protects pregnancy from overt OS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

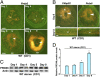
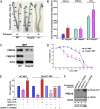
References
-
- Margalioth EJ, Ben-Chetrit A, Gal M, Eldar-Geva T. Investigation and treatment of repeated implantation failure following IVF-ET. Hum Reprod. 2006;21:3036–3043. - PubMed
-
- Dey SK, et al. Molecular cues to implantation. Endocr Rev. 2004;25:341–373. - PubMed
-
- Lydon JP, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

