Flecainide increases Kir2.1 currents by interacting with cysteine 311, decreasing the polyamine-induced rectification
- PMID: 20713726
- PMCID: PMC2932566
- DOI: 10.1073/pnas.1004021107
Flecainide increases Kir2.1 currents by interacting with cysteine 311, decreasing the polyamine-induced rectification
Abstract
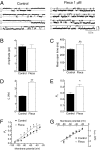
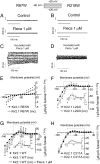
Both increase and decrease of cardiac inward rectifier current (I(K1)) are associated with severe cardiac arrhythmias. Flecainide, a widely used antiarrhythmic drug, exhibits ventricular proarrhythmic effects while effectively controlling ventricular arrhythmias associated with mutations in the gene encoding Kir2.1 channels that decrease I(K1) (Andersen syndrome). Here we characterize the electrophysiological and molecular basis of the flecainide-induced increase of the current generated by Kir2.1 channels (I(Kir2.1)) and I(K1) recorded in ventricular myocytes. Flecainide increases outward I(Kir2.1) generated by homotetrameric Kir2.1 channels by decreasing their affinity for intracellular polyamines, which reduces the inward rectification of the current. Flecainide interacts with the HI loop of the cytoplasmic domain of the channel, Cys311 being critical for the effect. This explains why flecainide does not increase I(Kir2.2) and I(Kir2.3), because Kir2.2 and Kir2.3 channels do not exhibit a Cys residue at the equivalent position. We further show that incubation with flecainide increases expression of functional Kir2.1 channels in the membrane, an effect also determined by Cys311. Indeed, flecainide pharmacologically rescues R67W, but not R218W, channel mutations found in Andersen syndrome patients. Moreover, our findings provide noteworthy clues about the structural determinants of the C terminus cytoplasmic domain of Kir2.1 channels involved in the control of gating and rectification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


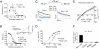

References
-
- Wang Z, Yue L, White M, Pelletier G, Nattel S. Differential distribution of inward rectifier potassium channel transcripts in human atrium versus ventricle. Circulation. 1998;98:2422–2428. - PubMed
-
- Lu Z. Mechanism of rectification in inward-rectifier K+ channels. Annu Rev Physiol. 2004;66:103–129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

