Amphiphile regulation of ion channel function by changes in the bilayer spring constant
- PMID: 20713738
- PMCID: PMC2932597
- DOI: 10.1073/pnas.1007455107
Amphiphile regulation of ion channel function by changes in the bilayer spring constant
Abstract
Many drugs are amphiphiles that, in addition to binding to a particular target protein, adsorb to cell membrane lipid bilayers and alter intrinsic bilayer physical properties (e.g., bilayer thickness, monolayer curvature, and elastic moduli). Such changes can modulate membrane protein function by altering the energetic cost (DeltaG(bilayer)) of bilayer deformations associated with protein conformational changes that involve the protein-bilayer interface. But amphiphiles have complex effects on the physical properties of lipid bilayers, meaning that the net change in DeltaG(bilayer) cannot be predicted from measurements of isolated changes in such properties. Thus, the bilayer contribution to the promiscuous regulation of membrane proteins by drugs and other amphiphiles remains unknown. To overcome this problem, we use gramicidin A (gA) channels as molecular force probes to measure the net effect of amphiphiles, at concentrations often used in biological research, on the bilayer elastic response to a change in the hydrophobic length of an embedded protein. The effects of structurally diverse amphiphiles can be described by changes in a phenomenological bilayer spring constant (H(B)) that summarizes the bilayer elastic properties, as sensed by a bilayer-spanning protein. Amphiphile-induced changes in H(B), measured using gA channels of a particular length, quantitatively predict changes in lifetime for channels of a different length--as well as changes in the inactivation of voltage-dependent sodium channels in living cells. The use of gA channels as molecular force probes provides a tool for quantitative, predictive studies of bilayer-mediated regulation of membrane protein function by amphiphiles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
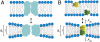


 , right axis) vs. corresponding effects on channels formed by 15-residue subunits (bottom or top axis). (A) Effects of TX100, capsaicin, capsazepine, daidzein, genistein, phloretin, or GsMTx4 in DPhPC/n-decane bilayers (except for TX100, results from refs. , and 22). A subset of the results was published previously (5). (B) Effects of TX100, curcumin, docosahexaenoic acid (DHA), 2,3-butanedione monoxime (BDM), reduced Triton X-100 (rTX100), Z-Gly-D-Phe (ZGdF), and Z-Gly-Phe (ZGF) (at pH 7 or pH 4) and Z-D-Phe-Phe-Gly (ZdFFG, at pH 7) in DOPC/n-decane bilayers (except for TX100, results from refs. –28). (C) Superimposition of results obtained using DPhPC (red) or DOPC (black). (D) Distribution of
, right axis) vs. corresponding effects on channels formed by 15-residue subunits (bottom or top axis). (A) Effects of TX100, capsaicin, capsazepine, daidzein, genistein, phloretin, or GsMTx4 in DPhPC/n-decane bilayers (except for TX100, results from refs. , and 22). A subset of the results was published previously (5). (B) Effects of TX100, curcumin, docosahexaenoic acid (DHA), 2,3-butanedione monoxime (BDM), reduced Triton X-100 (rTX100), Z-Gly-D-Phe (ZGdF), and Z-Gly-Phe (ZGF) (at pH 7 or pH 4) and Z-D-Phe-Phe-Gly (ZdFFG, at pH 7) in DOPC/n-decane bilayers (except for TX100, results from refs. –28). (C) Superimposition of results obtained using DPhPC (red) or DOPC (black). (D) Distribution of  for individual amphiphiles using DPhPC (red) or DOPC (black). Mean ± SEM (n≥3) or ± range (n = 2).
for individual amphiphiles using DPhPC (red) or DOPC (black). Mean ± SEM (n≥3) or ± range (n = 2).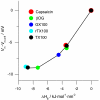
Similar articles
-
Genistein can modulate channel function by a phosphorylation-independent mechanism: importance of hydrophobic mismatch and bilayer mechanics.Biochemistry. 2003 Nov 25;42(46):13646-58. doi: 10.1021/bi034887y. Biochemistry. 2003. PMID: 14622011
-
Regulation of sodium channel function by bilayer elasticity: the importance of hydrophobic coupling. Effects of Micelle-forming amphiphiles and cholesterol.J Gen Physiol. 2004 May;123(5):599-621. doi: 10.1085/jgp.200308996. J Gen Physiol. 2004. PMID: 15111647 Free PMC article.
-
Linear rate-equilibrium relations arising from ion channel-bilayer energetic coupling.Proc Natl Acad Sci U S A. 2011 Aug 2;108(31):12717-22. doi: 10.1073/pnas.1103192108. Epub 2011 Jul 18. Proc Natl Acad Sci U S A. 2011. PMID: 21768343 Free PMC article.
-
Single-molecule methods for monitoring changes in bilayer elastic properties.Methods Mol Biol. 2007;400:543-70. doi: 10.1007/978-1-59745-519-0_37. Methods Mol Biol. 2007. PMID: 17951759 Review.
-
Lipid bilayer regulation of membrane protein function: gramicidin channels as molecular force probes.J R Soc Interface. 2010 Mar 6;7(44):373-95. doi: 10.1098/rsif.2009.0443. Epub 2009 Nov 25. J R Soc Interface. 2010. PMID: 19940001 Free PMC article. Review.
Cited by
-
The Antimicrobial Peptide Gramicidin S Enhances Membrane Adsorption and Ion Pore Formation Potency of Chemotherapy Drugs in Lipid Bilayers.Membranes (Basel). 2021 Mar 30;11(4):247. doi: 10.3390/membranes11040247. Membranes (Basel). 2021. PMID: 33808204 Free PMC article.
-
Anionic nanoparticle-induced perturbation to phospholipid membranes affects ion channel function.Proc Natl Acad Sci U S A. 2020 Nov 10;117(45):27854-27861. doi: 10.1073/pnas.2004736117. Epub 2020 Oct 26. Proc Natl Acad Sci U S A. 2020. PMID: 33106430 Free PMC article.
-
Computational modeling of membrane proteins.Proteins. 2015 Jan;83(1):1-24. doi: 10.1002/prot.24703. Epub 2014 Nov 19. Proteins. 2015. PMID: 25355688 Free PMC article. Review.
-
The bile acid-sensitive ion channel (BASIC) is activated by alterations of its membrane environment.PLoS One. 2014 Oct 31;9(10):e111549. doi: 10.1371/journal.pone.0111549. eCollection 2014. PLoS One. 2014. PMID: 25360526 Free PMC article.
-
Phytochemicals perturb membranes and promiscuously alter protein function.ACS Chem Biol. 2014 Aug 15;9(8):1788-98. doi: 10.1021/cb500086e. Epub 2014 Jun 17. ACS Chem Biol. 2014. PMID: 24901212 Free PMC article.
References
-
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972;24:583–655. - PubMed
-
- Sackmann E. In: Biological Membranes. Chapman D, editor. London: Academic; 1984. pp. 105–143.
-
- Seddon AM, et al. Drug interactions with lipid membranes. Chem Soc Rev. 2009;38:2509–2519. - PubMed
-
- Lundbæk JA. Regulation of membrane protein function by lipid bilayer elasticity: A single molecule technology to measure the bilayer properties experienced by an embedded protein. J Phys Condens Matt. 2006;18:S1305–S1344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

