Randomized, phase II study of the insulin-like growth factor-1 receptor inhibitor IMC-A12, with or without cetuximab, in patients with cetuximab- or panitumumab-refractory metastatic colorectal cancer
- PMID: 20713879
- PMCID: PMC3296668
- DOI: 10.1200/JCO.2010.30.4154
Randomized, phase II study of the insulin-like growth factor-1 receptor inhibitor IMC-A12, with or without cetuximab, in patients with cetuximab- or panitumumab-refractory metastatic colorectal cancer
Abstract
Purpose: To evaluate the safety and efficacy of IMC-A12, a human monoclonal antibody (mAb) that blocks insulin-like growth factor receptor-1 (IGF-1R), as monotherapy or in combination with cetuximab in patients with metastatic refractory anti-epidermal growth factor receptor (EGFR) mAb colorectal cancer.
Methods: A randomized, phase II study was performed in which patients in arm A received IMC-A12 10 mg/kg intravenously (IV) every 2 weeks, while patients in arm B received this same dose of IMC-A12 plus cetuximab 500 mg/m(2) IV every 2 weeks. Subsequently, arm C (same combination treatment as arm B) was added to include patients who had disease control on a prior anti-EGFR mAb and wild-type KRAS tumors. Archived pretreatment tumor tissue was obtained when possible for KRAS, PIK3CA, and BRAF genotyping, and immunohistochemistry was obtained for pAKT as well as IGF-1R.
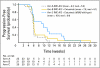
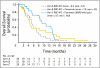
Results: Overall, 64 patients were treated (median age, 61 years; range, 40 to 84 years): 23 patients in arm A, 21 in arm B, and 20 in arm C. No antitumor activity was seen in the 23 patients treated with IMC-A12 monotherapy. Of the 21 patients randomly assigned to IMC-A12 plus cetuximab, one patient (with KRAS wild type) achieved a partial response, with disease control lasting 6.5 months. Arm C (all patients with KRAS wild type), however, showed no additional antitumor activity. Serious adverse events thought possibly related to IMC-A12 included a grade 2 infusion-related reaction (2%; one of 64 patients), thrombocytopenia (2%; one of 64 patients), grade 3 hyperglycemia (2%; one of 64 patients), and grade 1 pyrexia (2%, one of 64 patients).
Conclusion: IMC-A12 alone or in combination with cetuximab was insufficient to warrant additional study in patients with colorectal cancer refractory to EGFR inhibitors.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Wang Y, Sun Y. Insulin-like growth factor receptor-1 as an anti-cancer target: Blocking transformation and inducing apoptosis. Curr Cancer Drug Targets. 2002;2:191–207. - PubMed
-
- Moschos SJ, Mantzoros CS. The role of the IGF system in cancer: From basic to clinical studies and clinical applications. Oncology. 2002;63:317–332. - PubMed
-
- Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. - PubMed
-
- Burtrum D, Zhu Z, Lu D, et al. A fully human monoclonal antibody to the insulin-like growth factor I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer Res. 2003;63:8912–8921. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous