Ethylenecarbodiimide-treated splenocytes carrying male CD4 epitopes confer histocompatibility Y chromosome antigen transplant protection by inhibiting CD154 upregulation
- PMID: 20713889
- PMCID: PMC2933307
- DOI: 10.4049/jimmunol.1000802
Ethylenecarbodiimide-treated splenocytes carrying male CD4 epitopes confer histocompatibility Y chromosome antigen transplant protection by inhibiting CD154 upregulation
Abstract
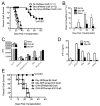
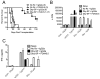
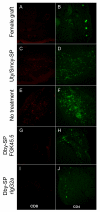
In humans and certain strains of laboratory mice, male tissue is recognized as nonself and destroyed by the female immune system via recognition of histocompatibility Y chromosome Ag (Hya). Male tissue destruction is thought to be accomplished by CTLs in a helper-dependent manner. We show that graft protection induced with the immunodominant Hya-encoded CD4 epitope (Dby) attached to female splenic leukocytes (Dby-SPs) with the chemical cross-linker ethylenecarbodiimide significantly, and often indefinitely, prolongs the survival of male skin graft transplants in an Ag-specific manner. In contrast, treatments with the Hya CD8 epitopes (Uty-/Smcy-SPs) failed to prolong graft survival. Dby-SP-tolerized CD4(+) T cells fail to proliferate, secrete IFN-gamma, or effectively prime a CD8 response in recipients of male grafts. Ag-coupled splenocyte treatment is associated with defective CD40-CD40L interactions as demonstrated by the observation that CD4 cells from treated animals exhibit a defect in CD40L upregulation following in vitro Ag challenge. Furthermore, treatment with an agonistic anti-CD40 Ab at the time of transplantation abrogates protection from graft rejection. Interestingly, anti-CD40 treatment completely restores the function of Dby-specific CD4 cells but not Uty- or Smcy-specific CD8 cells.
Figures





References
-
- Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat. Rev. Immunol. 2007;7:665–677. - PubMed
-
- Gonsette RE. Compared benefit of approved and experimental immunosuppressive therapeutic approaches in multiple sclerosis. Expert. Opin. Pharmacother. 2007;8:1103–1116. - PubMed
-
- Moreau T, Coles A, Wing M, Isaacs J, Hale G, Waldmann H, Compston A. Transient increase in symptoms associated with cytokine release in patients with multiple sclerosis. Brain. 1996;119:225–237. - PubMed
-
- Vanderlugt CL, Eagar TN, Neville KL, Nikcevich KM, Bluestone JA, Miller SD. Pathologic role and temporal appearance of newly emerging autoepitopes in relapsing experimental autoimmune encephalomyelitis. J. Immunol. 2000;164:670–678. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

