Epigenetic basis for aberrant upregulation of autoantigen genes in humans with ANCA vasculitis
- PMID: 20714105
- PMCID: PMC2929711
- DOI: 10.1172/JCI40034
Epigenetic basis for aberrant upregulation of autoantigen genes in humans with ANCA vasculitis
Abstract
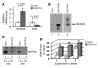
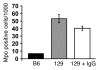
Antineutrophil cytoplasmic autoantibody (ANCA) causes vascular injury that leads to small-vessel vasculitis. Patients with ANCA aberrantly express neutrophil granule-encoding genes, including 2 that encode autoantigens: proteinase 3 (PR3) and myeloperoxidase (MPO). To uncover a potential transcriptional regulatory mechanism for PR3 and MPO disrupted in patients with ANCA vasculitis, we examined the PR3 and MPO loci in neutrophils from ANCA patients and healthy control individuals for epigenetic modifications associated with gene silencing. We found that levels of the chromatin modification H3K27me3, which is associated with gene silencing, were depleted at PR3 and MPO loci in ANCA patients compared with healthy controls. Interestingly, in both patients and controls, DNA was unmethylated at a CpG island in PR3, whereas in healthy controls, DNA was methylated at a CpG island in MPO. Consistent with decreased levels of H3K27me3, JMJD3, the demethylase specific for H3K27me3, was preferentially expressed in ANCA patients versus healthy controls. In addition, we describe a mechanism for recruiting the H3K27 methyltransferase enhancer of zeste homolog 2 (EZH2) to PR3 and MPO loci mediated by RUNX3. RUNX3 message was decreased in patients compared with healthy controls, and may also be under epigenetic control. DNA methylation was increased at the RUNX3 promoter in ANCA patients. These data indicate that epigenetic modifications associated with gene silencing are perturbed at ANCA autoantigen-encoding genes, potentially contributing to inappropriate expression of PR3 and MPO in ANCA patients.
Figures






Comment in
-
Vasculitis syndromes: Silence is golden as epigenetic mechanisms are blamed for autoantigen expression in ANCA vasculitis.Nat Rev Rheumatol. 2010 Nov;6(11):614. doi: 10.1038/nrrheum.2010.168. Nat Rev Rheumatol. 2010. PMID: 21064238 No abstract available.
Similar articles
-
Histone modification signature at myeloperoxidase and proteinase 3 in patients with anti-neutrophil cytoplasmic autoantibody-associated vasculitis.Clin Epigenetics. 2016 Aug 12;8:85. doi: 10.1186/s13148-016-0251-0. eCollection 2016. Clin Epigenetics. 2016. PMID: 27752292 Free PMC article.
-
Gene-Specific DNA Methylation Changes Predict Remission in Patients with ANCA-Associated Vasculitis.J Am Soc Nephrol. 2017 Apr;28(4):1175-1187. doi: 10.1681/ASN.2016050548. Epub 2016 Nov 7. J Am Soc Nephrol. 2017. PMID: 27821628 Free PMC article.
-
Intermediate monocytes in ANCA vasculitis: increased surface expression of ANCA autoantigens and IL-1β secretion in response to anti-MPO antibodies.Sci Rep. 2015 Jul 7;5:11888. doi: 10.1038/srep11888. Sci Rep. 2015. PMID: 26149790 Free PMC article.
-
Recent pathogenetic advances in ANCA-associated vasculitis.Presse Med. 2015 Jun;44(6 Pt 2):e223-9. doi: 10.1016/j.lpm.2015.04.007. Epub 2015 May 29. Presse Med. 2015. PMID: 26033562 Review.
-
Pathogenesis of PR3-ANCA associated vasculitis.J Autoimmun. 2008 Feb-Mar;30(1-2):29-36. doi: 10.1016/j.jaut.2007.11.005. Epub 2007 Dec 26. J Autoimmun. 2008. PMID: 18162369 Review.
Cited by
-
How anti-neutrophil cytoplasmic autoantibodies activate neutrophils.Clin Exp Immunol. 2012 Sep;169(3):220-8. doi: 10.1111/j.1365-2249.2012.04615.x. Clin Exp Immunol. 2012. PMID: 22861361 Free PMC article. Review.
-
Current State of Precision Medicine in Primary Systemic Vasculitides.Front Immunol. 2019 Dec 17;10:2813. doi: 10.3389/fimmu.2019.02813. eCollection 2019. Front Immunol. 2019. PMID: 31921111 Free PMC article. Review.
-
Regulation and Role of EZH2 in Cancer.Cancer Res Treat. 2014 Jul;46(3):209-22. doi: 10.4143/crt.2014.46.3.209. Epub 2014 Jul 15. Cancer Res Treat. 2014. PMID: 25038756 Free PMC article.
-
Dysregulation of autoantigen genes in ANCA-associated vasculitis involves alternative transcripts and new protein synthesis.J Am Soc Nephrol. 2015 Feb;26(2):390-9. doi: 10.1681/ASN.2013101092. Epub 2014 Jul 24. J Am Soc Nephrol. 2015. PMID: 25060059 Free PMC article.
-
Distinguishing active from quiescent disease in ANCA-associated vasculitis using attenuated total reflection Fourier-transform infrared spectroscopy.Sci Rep. 2021 May 11;11(1):9981. doi: 10.1038/s41598-021-89344-8. Sci Rep. 2021. PMID: 33976282 Free PMC article.
References
-
- Bansal PJ, Tobin MC. Neonatal microscopic polyangiitis secondary to transfer of maternal myeloperoxidase-antineutrophil cytoplasmic antibody resulting in neonatal pulmonary hemorrhage and renal involvement. Ann Allergy Asthma Immunol. 2004;93(4):398–401. doi: 10.1016/S1081-1206(10)61400-7. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

