Activated protein C targets CD8+ dendritic cells to reduce the mortality of endotoxemia in mice
- PMID: 20714108
- PMCID: PMC2929901
- DOI: 10.1172/JCI42629
Activated protein C targets CD8+ dendritic cells to reduce the mortality of endotoxemia in mice
Abstract
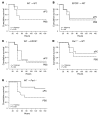
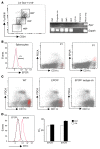
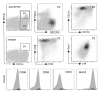
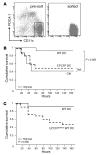
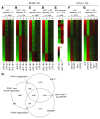
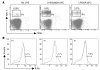
Activated protein C (aPC) therapy reduces mortality in adult patients with severe sepsis. In mouse endotoxemia and sepsis models, mortality reduction requires the cell signaling function of aPC, mediated through protease-activated receptor-1 (PAR1) and endothelial protein C receptor (EPCR; also known as Procr). Candidate cellular targets of aPC include vascular endothelial cells and leukocytes. Here, we show that expression of EPCR and PAR1 on hematopoietic cells is required in mice for an aPC variant that mediates full cell signaling activity but only minimal anticoagulant function (5A-aPC) to reduce the mortality of endotoxemia. Expression of EPCR in mature murine immune cells was limited to a subset of CD8+ conventional dendritic cells. Adoptive transfer of splenic CD11chiPDCA-1- dendritic cells from wild-type mice into animals with hematopoietic EPCR deficiency restored the therapeutic efficacy of aPC, whereas transfer of EPCR-deficient CD11chi dendritic cells or wild-type CD11chi dendritic cells depleted of EPCR+ cells did not. In addition, 5A-aPC inhibited the inflammatory response of conventional dendritic cells independent of EPCR and suppressed IFN-gamma production by natural killer-like dendritic cells. These data reveal an essential role for EPCR and PAR1 on hematopoietic cells, identify EPCR-expressing dendritic immune cells as a critical target of aPC therapy, and document EPCR-independent antiinflammatory effects of aPC on innate immune cells.
Figures
Comment in
-
New players in the sepsis-protective activated protein C pathway.J Clin Invest. 2010 Sep;120(9):3084-7. doi: 10.1172/JCI44266. Epub 2010 Aug 16. J Clin Invest. 2010. PMID: 20714106 Free PMC article.
Similar articles
-
Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C.J Exp Med. 2007 Oct 1;204(10):2439-48. doi: 10.1084/jem.20070404. Epub 2007 Sep 24. J Exp Med. 2007. PMID: 17893198 Free PMC article.
-
The efficacy of activated protein C in murine endotoxemia is dependent on integrin CD11b.J Clin Invest. 2010 Jun;120(6):1971-80. doi: 10.1172/JCI40380. Epub 2010 May 10. J Clin Invest. 2010. PMID: 20458145 Free PMC article.
-
Protection of vascular barrier integrity by activated protein C in murine models depends on protease-activated receptor-1.Thromb Haemost. 2009 Apr;101(4):724-33. doi: 10.1160/th08-10-0632. Thromb Haemost. 2009. PMID: 19350118 Free PMC article.
-
Endothelial cell protein C receptor-dependent signaling.Curr Opin Hematol. 2018 May;25(3):219-226. doi: 10.1097/MOH.0000000000000416. Curr Opin Hematol. 2018. PMID: 29461258 Free PMC article. Review.
-
The occupancy of endothelial protein C receptor by its ligand modulates the par-1 dependent signaling specificity of coagulation proteases.IUBMB Life. 2011 Jun;63(6):390-6. doi: 10.1002/iub.447. Epub 2011 Mar 24. IUBMB Life. 2011. PMID: 21438119 Free PMC article. Review.
Cited by
-
Overexpression of the endothelial protein C receptor is detrimental during pneumonia-derived gram-negative sepsis (Melioidosis).PLoS Negl Trop Dis. 2013 Jul 11;7(7):e2306. doi: 10.1371/journal.pntd.0002306. Print 2013. PLoS Negl Trop Dis. 2013. PMID: 23875041 Free PMC article.
-
EPCR-PAR1 biased signaling regulates perfusion recovery and neovascularization in peripheral ischemia.JCI Insight. 2022 Jul 22;7(14):e157701. doi: 10.1172/jci.insight.157701. JCI Insight. 2022. PMID: 35700057 Free PMC article.
-
Coagulation factor V mediates inhibition of tissue factor signaling by activated protein C in mice.Blood. 2015 Nov 19;126(21):2415-23. doi: 10.1182/blood-2015-05-644401. Epub 2015 Sep 4. Blood. 2015. PMID: 26341257 Free PMC article.
-
Activated protein C modulates T-cell metabolism and epigenetic FOXP3 induction via α-ketoglutarate.Blood Adv. 2023 Sep 12;7(17):5055-5068. doi: 10.1182/bloodadvances.2023010083. Blood Adv. 2023. PMID: 37315174 Free PMC article.
-
EPCR deficiency ameliorates inflammatory arthritis in mice by suppressing the activation and migration of T cells and dendritic cells.Rheumatology (Oxford). 2024 Feb 1;63(2):571-580. doi: 10.1093/rheumatology/kead230. Rheumatology (Oxford). 2024. PMID: 37228024 Free PMC article.
References
-
- Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109(8):3161–3172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL093388/HL/NHLBI NIH HHS/United States
- R33 AI080557/AI/NIAID NIH HHS/United States
- HL31950/HL/NHLBI NIH HHS/United States
- R01 HL052246/HL/NHLBI NIH HHS/United States
- R01 AI078713/AI/NIAID NIH HHS/United States
- R56 AI078713/AI/NIAID NIH HHS/United States
- HL073750/HL/NHLBI NIH HHS/United States
- P01 HL031950/HL/NHLBI NIH HHS/United States
- R01 HL093388/HL/NHLBI NIH HHS/United States
- AI078713/AI/NIAID NIH HHS/United States
- AI080557/AI/NIAID NIH HHS/United States
- P01 HL073750/HL/NHLBI NIH HHS/United States
- R21 AI080557/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

