Interactions of corticotropin-releasing factor, urocortin and citalopram in a primate model of stress-induced amenorrhea
- PMID: 20714124
- PMCID: PMC3025882
- DOI: 10.1159/000319257
Interactions of corticotropin-releasing factor, urocortin and citalopram in a primate model of stress-induced amenorrhea
Abstract
Background/aims: We established a cynomolgus macaque model of stress-induced amenorrhea in which the application of combined metabolic and psychosocial stress suppressed ovulation in stress-sensitive (SS) individuals, but not in highly stress-resilient (HSR) individuals. We previously reported that SS monkeys have deficits in global serotonin release and serotonin-related gene expression in the raphe nucleus, and that administration of the selective serotonin reuptake inhibitor S-citalopram increased estrogen and progesterone production in SS monkeys. In this study, we questioned whether there was a difference in corticotropin-releasing factor (CRF) or urocortin (UCN) stress-related peptide systems in the midbrain raphe region when HSR and SS monkeys treated with placebo or S-citalopram are compared.
Methods: Monkeys characterized as HSR or SS were administered placebo or S-citalopram for 15 weeks. CRF fibers in the dorsal raphe were detected with an antibody against human CRF. UCN1 fibers were immunostained in an area rostral to the dorsal raphe. The fibers were quantified by stereology and analyzed by two-way ANOVA. UCN1 cell bodies were counted in the supraoculomotor area near the Edinger-Westphal nucleus.
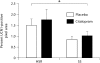
Results: S-citalopram significantly decreased the CRF fiber density in SS animals, but not in HSR animals. SS monkeys had a significantly lower UCN1 fiber density compared to HSR monkeys, but S-citalopram treatment did not alter the UCN1 fiber density. SS animals treated with S-citalopram tended to have a higher number of UCN1-positive cell bodies than the other groups.
Conclusion: S-citalopram decreased CRF fiber density and appears to increase the production of UCN1 in SS individuals, indicating the likelihood that serotonin is involved in regulating CRF and UCN1 in individuals who are sensitive to the effects of serotonin.
Copyright © 2010 S. Karger AG, Basel.
Figures

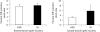
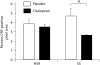




Similar articles
-
The effect of citalopram on midbrain CRF receptors 1 and 2 in a primate model of stress-induced amenorrhea.Reprod Sci. 2012 Jun;19(6):623-32. doi: 10.1177/1933719111430992. Epub 2012 Mar 12. Reprod Sci. 2012. PMID: 22412189 Free PMC article.
-
The effect of short moderate stress on the midbrain corticotropin-releasing factor system in a macaque model of functional hypothalamic amenorrhea.Fertil Steril. 2013 Oct;100(4):1111-21. doi: 10.1016/j.fertnstert.2013.05.052. Epub 2013 Jul 10. Fertil Steril. 2013. PMID: 23849846 Free PMC article.
-
Effects of citalopram on serotonin and CRF systems in the midbrain of primates with differences in stress sensitivity.J Chem Neuroanat. 2011 Jul;41(4):200-18. doi: 10.1016/j.jchemneu.2011.05.010. Epub 2011 Jun 6. J Chem Neuroanat. 2011. PMID: 21683135 Free PMC article. Review.
-
Stress sensitive female macaques have decreased fifth Ewing variant (Fev) and serotonin-related gene expression that is not reversed by citalopram.Neuroscience. 2009 Dec 1;164(2):676-91. doi: 10.1016/j.neuroscience.2009.08.010. Epub 2009 Aug 8. Neuroscience. 2009. PMID: 19671441 Free PMC article.
-
Contribution of Urocortin to the Development of Excessive Drinking.Int Rev Neurobiol. 2017;136:275-291. doi: 10.1016/bs.irn.2017.06.007. Epub 2017 Aug 2. Int Rev Neurobiol. 2017. PMID: 29056154 Review.
Cited by
-
Exposure to prenatal psychobiological stress exerts programming influences on the mother and her fetus.Neuroendocrinology. 2012;95(1):7-21. doi: 10.1159/000327017. Epub 2011 Apr 15. Neuroendocrinology. 2012. PMID: 21494029 Free PMC article. Review.
-
The effect of citalopram on midbrain CRF receptors 1 and 2 in a primate model of stress-induced amenorrhea.Reprod Sci. 2012 Jun;19(6):623-32. doi: 10.1177/1933719111430992. Epub 2012 Mar 12. Reprod Sci. 2012. PMID: 22412189 Free PMC article.
-
Ovarian steroid regulation of the midbrain corticotropin releasing factor and urocortin systems in macaques.Neuroscience. 2010 Dec 15;171(3):893-909. doi: 10.1016/j.neuroscience.2010.08.059. Epub 2010 Sep 15. Neuroscience. 2010. PMID: 20833230 Free PMC article.
-
Large-scale transcriptome sequencing and gene analyses in the crab-eating macaque (Macaca fascicularis) for biomedical research.BMC Genomics. 2012 May 4;13:163. doi: 10.1186/1471-2164-13-163. BMC Genomics. 2012. PMID: 22554259 Free PMC article.
-
[Mental disorders and female infertility].Nervenarzt. 2012 Nov;83(11):1442-7. doi: 10.1007/s00115-012-3662-y. Nervenarzt. 2012. PMID: 23052895 Review. German.
References
-
- Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506–1512. - PubMed
-
- Reindollar RH, Novak M, Tho SP, McDonough PG. Adult-onset amenorrhea: a study of 262 patients. Am J Obstet Gynecol. 1986;155:531–543. - PubMed
-
- Berga SL, Loucks TL. Use of cognitive behavior therapy for functional hypothalamic amenorrhea. Ann NY Acad Sci. 2006;1092:114–129. - PubMed
-
- Marcus MD, Loucks TL, Berga SL. Psychological correlates of functional hypothalamic amenorrhea. Fertil Steril. 2001;76:310–316. - PubMed
-
- Williams NI, Berga SL, Cameron JL. Synergism between psychosocial and metabolic stressors: impact on reproductive function in cynomolgus monkeys. Am J Physiol Endocrinol Metab. 2007;293:E270–E276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

