Cyclin T1 overexpression induces malignant transformation and tumor growth
- PMID: 20714219
- PMCID: PMC3040930
- DOI: 10.4161/cc.9.15.12526
Cyclin T1 overexpression induces malignant transformation and tumor growth
Abstract
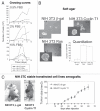
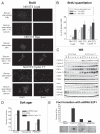
Human PTE Fb is a protein kinase composed by CDK9 and Cyclin T that controls the elongation phase of RNA Pol II. This complex also affects the activation and differentiation program of lymphoid cells. In this study we found that several head and neck tumor cell lines overexpress PTE Fb. We also established that Cyclin T1 is able to induce transformation in vitro, as we determined by foci and colony formation assays. Nu/nu mice s.c. injected with stable transfected Cyclin T1 cells (NIH 3T3 Cyclin T1) developed tumors faster than animals injected with control cells (NIH 3T3 beta-gal). In vitro, NIH 3T3 Cyclin T1 cells show increased proliferation and CDK4-Rb phosphorylation. Even more, silencing E2F1 expression (shRNA E2F1) in NIH 3T3 cells resulted in a dramatic inhibition of Cyclin T1-induced foci. All these data demonstrate for the first time the Cyclin T1 oncogenic function and suggest a role for this protein in controlling cell cycle probably via Rb/E2F1 pathway.
Figures



Comment in
-
Transcription elongation takes central stage: the P-TEFb connection.Cell Cycle. 2010 Aug 1;9(15):2933-4. doi: 10.4161/cc.9.15.12698. Epub 2010 Aug 1. Cell Cycle. 2010. PMID: 20703074 No abstract available.
-
P-TEFb joins the family of cdks in oncology, promotes cell growth of cancer cells.Cell Cycle. 2010 Aug 1;9(15):2935. doi: 10.4161/cc.9.15.12738. Epub 2010 Aug 1. Cell Cycle. 2010. PMID: 20703082 No abstract available.
References
-
- Morgan DO. Cyclin-dependent kinases: engines, clocks and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. - PubMed
-
- MacLachlan TK, Sang N, Giordano A. Cyclins, cyclin-dependent kinases and cdk inhibitors: implications in cell cycle control and cancer. Crit Rev Eukaryot Gene Expr. 1995;5:127–156. - PubMed
-
- Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. - PubMed
-
- Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–1828. - PubMed
-
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
