Chemistry of nitroquinolones and synthetic application to unnatural 1-methyl-2-quinolone derivatives
- PMID: 20714294
- PMCID: PMC6257690
- DOI: 10.3390/molecules15085174
Chemistry of nitroquinolones and synthetic application to unnatural 1-methyl-2-quinolone derivatives
Abstract
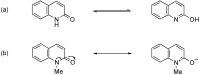
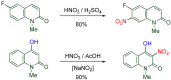
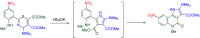
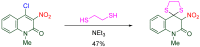
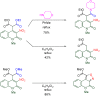
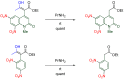
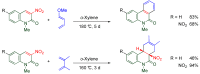
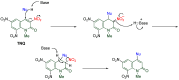
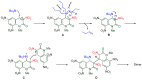
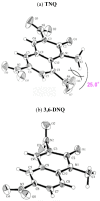
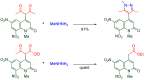
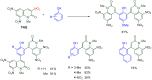
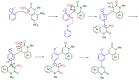
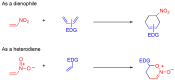
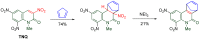
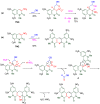
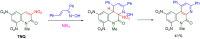
The 1-methyl-2-quinolone (MeQone) framework is often found in alkaloids and recently attention was drawn to unnatural MeQone derivatives with the aim of finding new biologically active compounds, however, low reactivity of the MeQone framework prevents the syntheses of versatile derivatives. A nitro group is one of the useful activating groups for this framework that enables a concise chemical transformation. Among nitroquinolones, 1-methyl-3,6,8-trinitro-2-quinolone (TNQ) exhibits unusual reactivity favoring region-selective cine-substitutions that afford 4-substituted 1-methyl-6,8-dinitro-2-quinolones upon treatment with nucleophilic reagents. Contrary to this, 1-methyl-3,6-dinitro-2-quinolone (3,6-DNQ) does not undergo any reaction under the same conditions. The unusual reactivity of TNQ is caused by steric repulsion between the methyl group at the 1-position and the nitro group at the 8-position, which distorts the MeQone framework. As a result, the pyridone ring of TNQ loses aromaticity and acts rather as an activated nitroalkene. Indeed, the pyridone moiety of TNQ undergoes cycloaddition with electron-rich alkenes or dienes under mild conditions, whereby a new fused ring is constructed on the [c]-face of the MeQone. Consequently, TNQ can be used as a new scaffold leading to versatile unnatural MeQone derivatives.
Figures
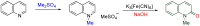
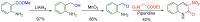
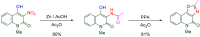

Similar articles
-
Ab initio and NBO studies of methyl internal rotation in 1-methyl-2(1H)-quinolinone: effect of aromatic substitution to 1-methyl-2(1H)-pyridone.J Mol Model. 2020 Apr 4;26(5):92. doi: 10.1007/s00894-020-04358-9. J Mol Model. 2020. PMID: 32246205
-
Recent Progress in Nitro-Promoted Direct Functionalization of Pyridones and Quinolones.Molecules. 2020 Feb 5;25(3):673. doi: 10.3390/molecules25030673. Molecules. 2020. PMID: 32033284 Free PMC article. Review.
-
Synthesis of unnatural 1-methyl-2-quinolone derivatives.Chem Pharm Bull (Tokyo). 2004 Nov;52(11):1334-8. doi: 10.1248/cpb.52.1334. Chem Pharm Bull (Tokyo). 2004. PMID: 15516757
-
Easily accessed nitroquinolones exhibiting potent and selective anti-tubercular activity.Eur J Med Chem. 2021 Mar 5;213:113207. doi: 10.1016/j.ejmech.2021.113207. Epub 2021 Jan 24. Eur J Med Chem. 2021. PMID: 33524688
-
Recent advances in the synthetic and medicinal perspective of quinolones: A review.Bioorg Chem. 2019 Nov;92:103291. doi: 10.1016/j.bioorg.2019.103291. Epub 2019 Sep 19. Bioorg Chem. 2019. PMID: 31561107 Review.
Cited by
-
Recent Advances in One-Pot Modular Synthesis of 2-Quinolones.Molecules. 2020 Nov 20;25(22):5450. doi: 10.3390/molecules25225450. Molecules. 2020. PMID: 33233747 Free PMC article. Review.
-
Ab initio and NBO studies of methyl internal rotation in 1-methyl-2(1H)-quinolinone: effect of aromatic substitution to 1-methyl-2(1H)-pyridone.J Mol Model. 2020 Apr 4;26(5):92. doi: 10.1007/s00894-020-04358-9. J Mol Model. 2020. PMID: 32246205
-
Comparison of Substituting Ability of Nitronate versus Enolate for Direct Substitution of a Nitro Group.Molecules. 2020 Apr 28;25(9):2048. doi: 10.3390/molecules25092048. Molecules. 2020. PMID: 32353998 Free PMC article.
-
Recent Progress in Nitro-Promoted Direct Functionalization of Pyridones and Quinolones.Molecules. 2020 Feb 5;25(3):673. doi: 10.3390/molecules25030673. Molecules. 2020. PMID: 32033284 Free PMC article. Review.
-
Biocatalytic and Chemo-Enzymatic Synthesis of Quinolines and 2-Quinolones by Monoamine Oxidase (MAO-N) and Horseradish Peroxidase (HRP) Biocatalysts.ACS Catal. 2023 Feb 22;13(5):3370-3378. doi: 10.1021/acscatal.2c05902. eCollection 2023 Mar 3. ACS Catal. 2023. PMID: 36910872 Free PMC article.
References
-
- Michael J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 1999;16:697–709. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

