Prevention of rotavirus gastroenteritis in infants and children: rotavirus vaccine safety, efficacy, and potential impact of vaccines
- PMID: 20714358
- PMCID: PMC2921258
- DOI: 10.2147/btt.s6530
Prevention of rotavirus gastroenteritis in infants and children: rotavirus vaccine safety, efficacy, and potential impact of vaccines
Abstract
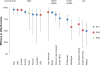
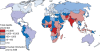
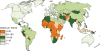
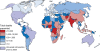
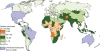
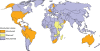
Rotavirus infection is the most common cause of severe gastroenteritis globally, with greater than 86% of deaths occurring in low-income and middle-income countries. There are two rotavirus vaccines currently licensed in the United States and prequalified by the World Health Organization. RV1 is a monovalent attenuated human rotavirus strain, given orally in two doses. RV5 is a pentavalent human-bovine reassortant rotavirus vaccine, given orally in three doses. A third rotavirus vaccine, LLV, is a lamb rotavirus strain given orally as a single dose, which is currently available only in China. RV1 and RV5 have been shown to be highly efficacious in developed countries, and initial results from trials in Africa and Asia are promising as well. At least three other vaccines are in development, which are being developed by manufacturers of developing countries. Further studies are needed to clarify issues including administration of oral rotavirus vaccines with breastfeeding and other oral vaccines, and alterations in dosing schedule. Using new data on global diarrheal burden, rotavirus is estimated to cause 390,000 deaths in children younger than 5 years. Should rotavirus vaccines be introduced in the routine immunization programs of all countries, a potential of 170,000 deaths could be prevented annually. The largest impact on mortality would be seen in low-income and middle-income countries, despite poor immunization coverage and lower efficacy. Therefore, international efforts are needed to ensure that rotavirus vaccines reach the populations with highest burden of rotavirus disease.
Keywords: gastroenteritis; mortality; rotavirus; vaccination.
Figures
References
-
- Glass RI, Kilgore PE, Holman RC, et al. The epidemiology of rotavirus diarrhea in the United States: surveillance and estimates of disease burden. J Infect Dis. 1996;174(Suppl 1):S5–S11. - PubMed
-
- Parashar UD, Burton A, Lanata C, et al. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis. 2009;200(Suppl 1):S9–S15. - PubMed
-
- Bernstein DI. Rotavirus overview. Pediatr Infect Dis J. 2009;28:S50–S53. - PubMed
LinkOut - more resources
Full Text Sources

