Neurotoxic alkaloids: saxitoxin and its analogs
- PMID: 20714432
- PMCID: PMC2920551
- DOI: 10.3390/md8072185
Neurotoxic alkaloids: saxitoxin and its analogs
Abstract
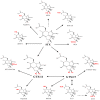
Saxitoxin (STX) and its 57 analogs are a broad group of natural neurotoxic alkaloids, commonly known as the paralytic shellfish toxins (PSTs). PSTs are the causative agents of paralytic shellfish poisoning (PSP) and are mostly associated with marine dinoflagellates (eukaryotes) and freshwater cyanobacteria (prokaryotes), which form extensive blooms around the world. PST producing dinoflagellates belong to the genera Alexandrium, Gymnodinium and Pyrodinium whilst production has been identified in several cyanobacterial genera including Anabaena, Cylindrospermopsis, Aphanizomenon Planktothrix and Lyngbya. STX and its analogs can be structurally classified into several classes such as non-sulfated, mono-sulfated, di-sulfated, decarbamoylated and the recently discovered hydrophobic analogs--each with varying levels of toxicity. Biotransformation of the PSTs into other PST analogs has been identified within marine invertebrates, humans and bacteria. An improved understanding of PST transformation into less toxic analogs and degradation, both chemically or enzymatically, will be important for the development of methods for the detoxification of contaminated water supplies and of shellfish destined for consumption. Some PSTs also have demonstrated pharmaceutical potential as a long-term anesthetic in the treatment of anal fissures and for chronic tension-type headache. The recent elucidation of the saxitoxin biosynthetic gene cluster in cyanobacteria and the identification of new PST analogs will present opportunities to further explore the pharmaceutical potential of these intriguing alkaloids.
Keywords: PSP; PSTs; STX; alkaloid analogs; neurotoxins; paralytic shellfish poisoning; paralytic shellfish toxins; saxitoxin.
Figures
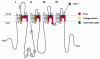
Similar articles
-
An overview on the marine neurotoxin, saxitoxin: genetics, molecular targets, methods of detection and ecological functions.Mar Drugs. 2013 Mar 27;11(4):991-1018. doi: 10.3390/md11040991. Mar Drugs. 2013. PMID: 23535394 Free PMC article. Review.
-
Paralytic shellfish poisoning toxin-producing cyanobacterium Aphanizomenon gracile in northeast Germany.Appl Environ Microbiol. 2010 Feb;76(4):1173-80. doi: 10.1128/AEM.02285-09. Epub 2010 Jan 4. Appl Environ Microbiol. 2010. PMID: 20048055 Free PMC article.
-
Saxitoxin: A Comprehensive Review of Its History, Structure, Toxicology, Biosynthesis, Detection, and Preventive Implications.Mar Drugs. 2025 Jul 2;23(7):277. doi: 10.3390/md23070277. Mar Drugs. 2025. PMID: 40710502 Free PMC article. Review.
-
Characterisation of the paralytic shellfish toxin biosynthesis gene clusters in Anabaena circinalis AWQC131C and Aphanizomenon sp. NH-5.BMC Biochem. 2009 Mar 30;10:8. doi: 10.1186/1471-2091-10-8. BMC Biochem. 2009. PMID: 19331657 Free PMC article.
-
Evolution and distribution of saxitoxin biosynthesis in dinoflagellates.Mar Drugs. 2013 Aug 8;11(8):2814-28. doi: 10.3390/md11082814. Mar Drugs. 2013. PMID: 23966031 Free PMC article. Review.
Cited by
-
From the sxtA4 Gene to Saxitoxin Production: What Controls the Variability Among Alexandrium minutum and Alexandrium pacificum Strains?Front Microbiol. 2021 Feb 24;12:613199. doi: 10.3389/fmicb.2021.613199. eCollection 2021. Front Microbiol. 2021. PMID: 33717003 Free PMC article.
-
Contamination Status and Acute Dietary Exposure Assessment of Paralytic Shellfish Toxins in Shellfish in the Dalian Area of the Yellow-Bohai Sea, China.Foods. 2024 Jan 23;13(3):361. doi: 10.3390/foods13030361. Foods. 2024. PMID: 38338497 Free PMC article.
-
Paralytic Shellfish Toxin Uptake, Assimilation, Depuration, and Transformation in the Southeast Asian Green-Lipped Mussel (Perna viridis).Toxins (Basel). 2019 Aug 9;11(8):468. doi: 10.3390/toxins11080468. Toxins (Basel). 2019. PMID: 31404969 Free PMC article.
-
Paralytic Shellfish Toxins in the Gastropod Concholepas concholepas: Variability, Toxin Profiles and Mechanisms for Toxicity Reduction.Mar Drugs. 2023 Jan 6;21(1):44. doi: 10.3390/md21010044. Mar Drugs. 2023. PMID: 36662217 Free PMC article.
-
Drugs as Chemical Weapons: Past and Perspectives.Toxics. 2023 Jan 4;11(1):52. doi: 10.3390/toxics11010052. Toxics. 2023. PMID: 36668778 Free PMC article. Review.
References
-
- Schantz EJ, Mold J, Stanger D, Shavel J, Riel F, Bowden J, Lynch J, Wyler R, Riegel B, Sommer H. Paralytic shellfish poison VI. A procedure for the isolation and purification of the poison from toxic clams and mussel tissues. J. Am. Chem. Soc. 1957;79:5230–5235.
-
- Anderson DM, Cembella AD, Hallegraeff GM. Physiological Ecology of Harmful Algal Blooms. 1st ed. Springer; Berlin, Germany: 1998. p. 662.
-
- Anderson DM, Glibert PM, Burkholder JM. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries. 2002;25:704–726.
-
- Sellner KG, Doucette GJ, Kirkpatrick GJ. Harmful algal blooms: Causes, impacts and detection. J. Ind. Microbiol. Biotechnol. 2003;30:383–406. - PubMed
-
- Zingone A, Enevoldsen HO. The diversity of harmful algal blooms: A challenge for science and management. Ocean Coast. Manage. 2000;43:725–748.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
