Antimetastatic efficacy of silibinin: molecular mechanisms and therapeutic potential against cancer
- PMID: 20714788
- PMCID: PMC3928361
- DOI: 10.1007/s10555-010-9237-0
Antimetastatic efficacy of silibinin: molecular mechanisms and therapeutic potential against cancer
Abstract
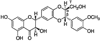
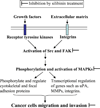
Cancer is a major health problem around the world. Research efforts in the last few decades have been successful in providing better and effective treatments against both early stage and localized cancer, but clinical options against advanced metastatic stage/s of cancer remain limited. The high morbidity and mortality in most of the cancers are attributed to their metastatic spread to distant organs. Due to its extreme clinical relevance, metastasis has been extensively studied and is now understood as a highly complex biological event that involves multiple steps including acquisition of invasiveness by cancer cells, intravasation into circulatory system, survival in the circulation, arrest in microvasculature, extravasation, and growth at distant organs. The increasing understanding of molecular underpinnings of these events has provided excellent opportunity to target metastasis especially through nontoxic and biologically effective nutraceuticals. Silibinin, a popular dietary supplement isolated from milk thistle seed extracts, is one such natural agent that has shown biological efficacy through pleiotropic mechanisms against a variety of cancers and is currently in clinical trials. Recent preclinical studies have also shown strong efficacy of silibinin to target cancer cell's migratory and invasive characteristics as well as their ability to metastasize to distant organs. Detailed mechanistic analyses revealed that silibinin targets signaling molecules involved in the regulation of epithelial-to-mesenchymal transition, proteases activation, adhesion, motility, invasiveness as well as the supportive tumor-microenvironment components, thereby inhibiting metastasis. Overall, the long history of human use, remarkable nontoxicity, and preclinical efficacy strongly favor the clinical use of silibinin against advanced metastatic cancers.
Figures


References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. - PubMed
-
- Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Mol Cancer Ther. 2007;6(10):2609–2617. - PubMed
-
- Tantivejkul K, Kalikin LM, Pienta KJ. Dynamic process of prostate cancer metastasis to bone. J Cell Biochem. 2004;91(4):706–717. - PubMed
-
- Hadaschik BA, Gleave ME. Therapeutic options for hormone-refractory prostate cancer in 2007. Urol Oncol. 2007;25(5):413–419. - PubMed
-
- Mehlen P, Puisieux A. Metastasis: A question of life or death. Nat Rev Cancer. 2006;6(6):449–458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

