Substrate stereo-specificity in tryptophan dioxygenase and indoleamine 2,3-dioxygenase
- PMID: 20715188
- PMCID: PMC2939288
- DOI: 10.1002/prot.22819
Substrate stereo-specificity in tryptophan dioxygenase and indoleamine 2,3-dioxygenase
Abstract
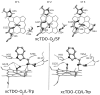
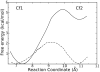
The first and rate-limiting step of the kynurenine pathway, in which tryptophan (Trp) is converted to N-formylkynurenine is catalyzed by two heme-containing proteins, Indoleamine 2,3-dioxygenase (IDO), and Tryptophan 2,3-dioxygenase (TDO). In mammals, TDO is found exclusively in liver tissue, IDO is found ubiquitously in all tissues. IDO has become increasingly popular in pharmaceutical research as it was found to be involved in many physiological situations, including immune escape of cancer. More importantly, small-molecule inhibitors of IDO are currently utilized in cancer therapy. One of the main concerns for the design of human IDO (hIDO) inhibitors is that they should be selective enough to avoid inhibition of TDO. In this work, we have used a combination of classical molecular dynamics (MD) and hybrid quantum-classical (QM/MM) methodologies to establish the structural basis that determine the differences in (a) the interactions of TDO and IDO with small ligands (CO/O(2)) and (b) the substrate stereo-specificity in hIDO and TDO. Our results indicate that the differences in small ligand bound structures of IDO and TDO arise from slight differences in the structure of the bound substrate complex. The results also show that substrate stereo-specificity of TDO is achieved by the perfect fit of L-Trp, but not D-Trp, which exhibits weaker interactions with the protein matrix. For hIDO, the presence of multiple stable binding conformations for L/D-Trp reveal the existence of a large and dynamic active site. Taken together, our data allow determination of key interactions useful for the future design of more potent hIDO-selective inhibitors.
© 2010 Wiley-Liss, Inc.
Figures
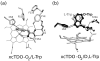


Similar articles
-
The first step of the dioxygenation reaction carried out by tryptophan dioxygenase and indoleamine 2,3-dioxygenase as revealed by quantum mechanical/molecular mechanical studies.J Biol Inorg Chem. 2010 Aug;15(6):811-23. doi: 10.1007/s00775-010-0646-x. Epub 2010 Apr 2. J Biol Inorg Chem. 2010. PMID: 20361220 Free PMC article.
-
The role of serine 167 in human indoleamine 2,3-dioxygenase: a comparison with tryptophan 2,3-dioxygenase.Biochemistry. 2008 Apr 22;47(16):4761-9. doi: 10.1021/bi702405a. Epub 2008 Mar 28. Biochemistry. 2008. PMID: 18370410
-
Oxidation of L-tryptophan in biology: a comparison between tryptophan 2,3-dioxygenase and indoleamine 2,3-dioxygenase.Biochem Soc Trans. 2009 Apr;37(Pt 2):408-12. doi: 10.1042/BST0370408. Biochem Soc Trans. 2009. PMID: 19290871 Review.
-
Human tryptophan dioxygenase: a comparison to indoleamine 2,3-dioxygenase.J Am Chem Soc. 2007 Dec 19;129(50):15690-701. doi: 10.1021/ja076186k. Epub 2007 Nov 21. J Am Chem Soc. 2007. PMID: 18027945
-
Heme-based dioxygenases: Structure, function and dynamics.J Inorg Biochem. 2024 Dec;261:112707. doi: 10.1016/j.jinorgbio.2024.112707. Epub 2024 Aug 30. J Inorg Biochem. 2024. PMID: 39217822 Review.
Cited by
-
Influence of tryptophan contained in 1-Methyl-Tryptophan on antimicrobial and immunoregulatory functions of indoleamine 2,3-dioxygenase.PLoS One. 2012;7(9):e44797. doi: 10.1371/journal.pone.0044797. Epub 2012 Sep 13. PLoS One. 2012. PMID: 23028625 Free PMC article.
-
Indoleamine 2,3-dioxygenase 1 (IDO1): an up-to-date overview of an eclectic immunoregulatory enzyme.FEBS J. 2022 Oct;289(20):6099-6118. doi: 10.1111/febs.16086. Epub 2021 Jun 30. FEBS J. 2022. PMID: 34145969 Free PMC article. Review.
-
Kynurenine and uric acid levels in chronic myeloid leukemia patients.Oncoimmunology. 2015 Mar 25;4(3):e992646. doi: 10.4161/2162402X.2014.992646. eCollection 2015 Mar. Oncoimmunology. 2015. PMID: 25949913 Free PMC article.
-
Kynurenic acid and 3-hydroxykynurenine production from D-kynurenine in mice.Brain Res. 2012 May 21;1455:1-9. doi: 10.1016/j.brainres.2012.03.026. Epub 2012 Mar 17. Brain Res. 2012. PMID: 22498176 Free PMC article.
-
Conformational Plasticity in Human Heme-Based Dioxygenases.J Am Chem Soc. 2021 Feb 3;143(4):1836-1845. doi: 10.1021/jacs.0c09970. Epub 2020 Dec 29. J Am Chem Soc. 2021. PMID: 33373218 Free PMC article.
References
-
- Sono M, Roach MP, Coulter ED, Dawson JH. Heme-Containing Oxygenases. Chem Rev. 1996;96(7):2841–2888. - PubMed
-
- Takikawa O. Biochemical and medical aspects of the indoleamine 2,3-dioxygenase-initiated L-tryptophan metabolism. Biochem Biophys Res Commun. 2005;338(1):12–19. - PubMed
-
- Forouhar F, Anderson JLR, Mowat CG, Vorobiev SM, Hussain A, Abashidze M, Bruckmann C, Thackray SJ, Seetharaman J, Tucker T, Xiao R, Ma LC, Zhao L, Acton TB, Montelione GT, Chapman SK, Tong L. Molecular insights into substrate recognition and catalysis by tryptophan 2,3-dioxygenase. Proc Natl Acad Sci U S A. 2007;104(2):473–478. - PMC - PubMed
-
- Zhang Y, Kang SA, Mukherjee T, Bale S, Crane BR, Begley TP, Ealick SE. Crystal structure and mechanism of tryptophan 2,3-dioxygenase, a heme enzyme involved in tryptophan catabolism and in quinolinate biosynthesis. Biochemistry. 2007;46(1):145–155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

