PEG-based hydrogels with collagen mimetic peptide-mediated and tunable physical cross-links
- PMID: 20715762
- PMCID: PMC3006224
- DOI: 10.1021/bm100465q
PEG-based hydrogels with collagen mimetic peptide-mediated and tunable physical cross-links
Abstract
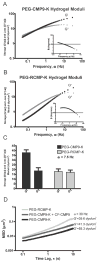
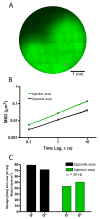
Mechanical properties of tissue scaffolds have major effects on the morphology and differentiation of cells. In contrast to two-dimensional substrates, local biochemical and mechanical properties of three-dimensional hydrogels are difficult to control due to the geometrical confinement. We designed synthetic 3D hydrogels featuring complexes of four-arm poly(ethylene glycol) (PEG) and collagen mimetic peptides (CMPs) that form hydrogels via physical cross-links mediated by thermally reversible triple helical assembly of CMPs. Here we present the fabrication of various PEG-CMP 3D hydrogels and their local mechanical properties determined by particle tracking microrheology. Results show that CMP mediated physical cross-links can be disrupted by altering the temperature of the gel or by adding free CMPs that compete for triple helix formation. This allowed modulation of both bulk and local stiffness as well as the creation of stiffness gradients within the PEG-CMP hydrogel, which demonstrates its potential as a novel scaffold for encoding physicochemical signals for tissue formation.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

