Converting GLX2-1 into an active glyoxalase II
- PMID: 20715794
- PMCID: PMC2939260
- DOI: 10.1021/bi1010865
Converting GLX2-1 into an active glyoxalase II
Abstract
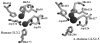
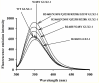
Arabidopsis thaliana glyoxalase 2-1 (GLX2-1) exhibits extensive sequence similarity with GLX2 enzymes but is catalytically inactive with SLG, the GLX2 substrate. In an effort to identify residues essential for GLX2 activity, amino acid residues were altered at positions 219, 246, 248, 325, and 328 in GLX2-1 to be the same as those in catalytically active human GLX2. The resulting enzymes were overexpressed, purified, and characterized using metal analyses, fluorescence spectroscopy, and steady-state kinetics to evaluate how these residues affect metal binding, structure, and catalysis. The R246H/N248Y double mutant exhibited low level S-lactoylglutathione hydrolase activity, while the R246H/N248Y/Q325R/R328K mutant exhibited a 1.5-2-fold increase in k(cat) and a decrease in K(m) as compared to the values exhibited by the double mutant. In contrast, the R246H mutant of GLX2-1 did not exhibit glyoxalase 2 activity. Zn(II)-loaded R246H GLX2-1 enzyme bound 2 equiv of Zn(II), and (1)H NMR spectra of the Co(II)-substituted analogue of this enzyme strongly suggest that the introduced histidine binds to Co(II). EPR studies indicate the presence of significant amounts a dinuclear metal ion-containing center. Therefore, an active GLX2 enzyme requires both the presence of a properly positioned metal center and significant nonmetal, enzyme-substrate contacts, with tyrosine 255 being particularly important.
Figures






Similar articles
-
Arabidopsis thaliana GLX2-1 contains a dinuclear metal binding site, but is not a glyoxalase 2.Biochem J. 2009 Jan 1;417(1):323-30. doi: 10.1042/BJ20081151. Biochem J. 2009. PMID: 18782082 Free PMC article.
-
Structural studies on a mitochondrial glyoxalase II.J Biol Chem. 2005 Dec 9;280(49):40668-75. doi: 10.1074/jbc.M509748200. Epub 2005 Oct 14. J Biol Chem. 2005. PMID: 16227621 Free PMC article.
-
The binding of iron and zinc to glyoxalase II occurs exclusively as di-metal centers and is unique within the metallo-beta-lactamase family.J Biol Inorg Chem. 2004 Jun;9(4):429-38. doi: 10.1007/s00775-004-0535-2. Epub 2004 Apr 6. J Biol Inorg Chem. 2004. PMID: 15067523
-
Bacterial glyoxalase enzymes.Semin Cell Dev Biol. 2011 May;22(3):285-92. doi: 10.1016/j.semcdb.2011.02.004. Epub 2011 Feb 15. Semin Cell Dev Biol. 2011. PMID: 21310258 Review.
-
Molecular enzymology of the glyoxalase system.Drug Metabol Drug Interact. 2008;23(1-2):13-27. doi: 10.1515/dmdi.2008.23.1-2.13. Drug Metabol Drug Interact. 2008. PMID: 18533362 Review.
Cited by
-
Characteristic Variations and Similarities in Biochemical, Molecular, and Functional Properties of Glyoxalases across Prokaryotes and Eukaryotes.Int J Mol Sci. 2017 Mar 30;18(4):250. doi: 10.3390/ijms18040250. Int J Mol Sci. 2017. PMID: 28358304 Free PMC article. Review.
-
Arabidopsis thaliana glyoxalase 2-1 is required during abiotic stress but is not essential under normal plant growth.PLoS One. 2014 Apr 23;9(4):e95971. doi: 10.1371/journal.pone.0095971. eCollection 2014. PLoS One. 2014. PMID: 24760003 Free PMC article.
References
-
- Thorson JS, Chapman E, Schultz PG. Analysis of Hydrogen Bonding Strengths in Proteins Using Unnatural Amino Acids. J. Am . Chem . Soc. 1995;117:9361–9362.
-
- Thornalley PJ. Pharmacology of Methylglyoxal: Formation, Modification of Proteins and Nucleic Acids, and Enzymatic Detoxification--A Role in Pathogenesis and Antiproliferative Chemotherapy. Gen. Pharmacol. 1996;27:565–573. - PubMed
-
- Thornalley PJ. Methylglyoxal, Glyoxalases and the Development of Diabetic Complications. Amino Acids. 1994;6:15–23. - PubMed
-
- Thornalley PJ. Modification of the Glyoxalase System in Disease Processes and Prospects for Therapeutic Strategies. Biochem. Soc. Trans. 1993;21:531–534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

