Association of serotype with risk of death due to pneumococcal pneumonia: a meta-analysis
- PMID: 20715907
- PMCID: PMC2927802
- DOI: 10.1086/655828
Association of serotype with risk of death due to pneumococcal pneumonia: a meta-analysis
Abstract
Background: The 92 capsular serotypes of Streptococcus pneumoniae differ greatly in nasopharyngeal carriage prevalence, invasiveness, and disease incidence. There has been some debate, though, regarding whether serotype independently affects the outcome of invasive pneumococcal disease (IPD). Published studies have shown variable results with regard to case-fatality ratios for specific serotypes and the role of host factors in affecting these relationships. We evaluated whether risk of death due to IPD is a stable serotype-associated property across studies and then compared the pooled effect estimates with epidemiologic and biological correlates.
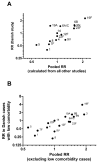
Methods: We performed a systematic review and meta-analysis of serotype-specific disease outcomes for patients with pneumonia and meningitis. Study-specific estimates of risk of death (risk ratio [RR]) were pooled from 9 studies that provided serotype-specific data on pneumonia and meningitis using a random-effects method with serotype 14 as the reference. Pooled RRs were compared with RRs from adults with low comorbidity scores to evaluate potential confounding by host factors.
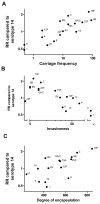
Results: Significant differences were found in the RR estimates among serotypes in patients with bacteremic pneumonia. Overall, serotypes 1, 7F, and 8 were associated with decreased RRs, and serotypes 3, 6A, 6B, 9N, and 19F were associated with increased RRs. Outcomes among meningitis patients did not differ significantly among serotypes. Serotypes with increased RRs had a high carriage prevalence, had low invasiveness, and were more heavily encapsulated in vitro.
Conclusions: These results suggest that IPD outcome, like other epidemiologic measures, is a stable serotype-associated property.
Conflict of interest statement
Conflicts of interest: SR has received travel grants from Wyeth; EAMS reports receiving unrestricted grants from Wyeth and Baxter for research, consulting fees for Wyeth and GlaxoSmithKline, lecturing fees from Wyeth and grant support from Wyeth and GlaxoSmithKline for vaccine studies. ML has received honoraria or consulting income from Pfizer, Novartis, and the Avian/Pandemic Flu Registry (Outcome Sciences), sponsored in part by Roche. KPK has received consulting and research support from Pfizer Vaccines, and consulting for Merck and Novartis. The rest of the authors declare no conflicts.
Figures

References
-
- Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Micro. 2008;6(4):288–301. - PubMed
-
- Bogaert D, de Groot R, Hermans PWM. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144–54. - PubMed
-
- Hausdorff William P, Bryant J, Paradiso Peter R, Siber George R. Which Pneumococcal Serogroups Cause the Most Invasive Disease: Implications for Conjugate Vaccine Formulation and Use, Part I. Clin Infect Dis. 2000;30(1):100–21. - PubMed
-
- O'Brien KL. Pneumococcal Regional Serotype Distribution for Pneumococcal AMC TPP. 2008. [cited; Available from: http://www.vaccineamc.org/files/TPP_Codebook.pdf.
-
- Brueggemann AB, Peto TEA, Crook DW, Butler JC, Kristinsson KG, Spratt BG. Temporal and Geographic Stability of the Serogroup-Specific Invasive Disease Potential of Streptococcus pneumoniae in Children. J Infect Dis. 2004;190(7):1203–11. - PubMed

