Membrane-directed molecular assembly of the neuronal SNARE complex
- PMID: 20716122
- PMCID: PMC3822491
- DOI: 10.1111/j.1582-4934.2010.01152.x
Membrane-directed molecular assembly of the neuronal SNARE complex
Abstract
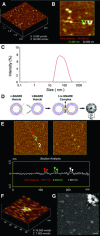
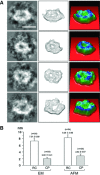
Since the discovery and implication of N-ethylmaleimide-sensitive factor (NSF)-attachment protein receptor (SNARE) proteins in membrane fusion almost two decades ago, there have been significant efforts to understand their involvement at the molecular level. In the current study, we report for the first time the molecular interaction between full-length recombinant t-SNAREs and v-SNARE present in opposing liposomes, leading to the assembly of a t-/v-SNARE ring complex. Using high-resolution electron microscopy, the electron density maps and 3D topography of the membrane-directed SNARE ring complex was determined at nanometre resolution. Similar to the t-/v-SNARE ring complex formed when 50 nm v-SNARE liposomes meet a t-SNARE-reconstituted planer membrane, SNARE rings are also formed when 50 nm diameter isolated synaptic vesicles (SVs) meet a t-SNARE-reconstituted planer lipid membrane. Furthermore, the mathematical prediction of the SNARE ring complex size with reasonable accuracy, and the possible mechanism of membrane-directed t-/v-SNARE ring complex assembly, was determined from the study. Therefore in the present study, using both lipososome-reconstituted recombinant t-/v-SNARE proteins, and native v-SNARE present in isolated SV membrane, the membrane-directed molecular assembly of the neuronal SNARE complex was determined for the first time and its size mathematically predicted. These results provide a new molecular understanding of the universal machinery and mechanism of membrane fusion in cells, having fundamental implications in human health and disease.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures



References
-
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–9. - PubMed

