Survival and function of mesenchymal stem cells (MSCs) depend on glucose to overcome exposure to long-term, severe and continuous hypoxia
- PMID: 20716129
- PMCID: PMC3823195
- DOI: 10.1111/j.1582-4934.2010.01138.x
Survival and function of mesenchymal stem cells (MSCs) depend on glucose to overcome exposure to long-term, severe and continuous hypoxia
Abstract
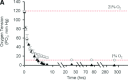
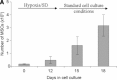
Use of mesenchymal stem cells (MSCs) has emerged as a potential new treatment for various diseases but has generated marginally successful results. A consistent finding of most studies is massive death of transplanted cells. The present study examined the respective roles of glucose and continuous severe hypoxia on MSC viability and function with respect to bone tissue engineering. We hereby demonstrate for the first time that MSCs survive exposure to long-term (12 days), severe (pO(2) < 1.5 mmHg) hypoxia, provided glucose is available. To this end, an in vitro model that mimics the hypoxic environment and cell-driven metabolic changes encountered by grafted sheep cells was established. In this model, the hallmarks of hypoxia (low pO(2) , hypoxia inducible factor-1α expression and anaerobic metabolism) were present. When conditions switched from hypoxic (low pO(2) ) to ischemic (low pO(2) and glucose depletion), MSCs exhibited shrinking, decreased cell viability and ATP content due to complete exhaustion of glucose at day 6; these results provided evidence that ischemia led to the observed massive cell death. Moreover, MSCs exposed to severe, continuous hypoxia, but without any glucose shortage, remained viable and maintained both their in vitro proliferative ability after simulation with blood reperfusion at day 12 and their in vivo osteogenic ability. These findings challenge the traditional view according to which severe hypoxia per se is responsible for the massive MSC death observed upon transplantation of these cells and provide evidence that MSCs are able to withstand exposure to severe, continuous hypoxia provided that a glucose supply is available.
Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd
Figures














Similar articles
-
Metabolic flexibility permits mesenchymal stem cell survival in an ischemic environment.Stem Cells. 2008 May;26(5):1325-36. doi: 10.1634/stemcells.2007-1072. Epub 2008 Feb 28. Stem Cells. 2008. PMID: 18308942
-
Inducible metabolic adaptation promotes mesenchymal stem cell therapy for ischemia: a hypoxia-induced and glycogen-based energy prestorage strategy.Arterioscler Thromb Vasc Biol. 2014 Apr;34(4):870-6. doi: 10.1161/ATVBAHA.114.303194. Epub 2014 Feb 20. Arterioscler Thromb Vasc Biol. 2014. PMID: 24558105
-
Hypoxia-preconditioned olfactory mucosa mesenchymal stem cells abolish cerebral ischemia/reperfusion-induced pyroptosis and apoptotic death of microglial cells by activating HIF-1α.Aging (Albany NY). 2020 Jun 7;12(11):10931-10950. doi: 10.18632/aging.103307. Epub 2020 Jun 7. Aging (Albany NY). 2020. PMID: 32507769 Free PMC article.
-
In vitro augmentation of mesenchymal stem cells viability in stressful microenvironments : In vitro augmentation of mesenchymal stem cells viability.Cell Stress Chaperones. 2015 Mar;20(2):237-51. doi: 10.1007/s12192-014-0560-1. Epub 2014 Dec 20. Cell Stress Chaperones. 2015. PMID: 25527070 Free PMC article. Review.
-
The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches.Tissue Eng Part B Rev. 2010 Apr;16(2):159-68. doi: 10.1089/ten.TEB.2009.0296. Tissue Eng Part B Rev. 2010. PMID: 19698058 Review.
Cited by
-
Hypoxia induces an undifferentiated phenotype of oral keratinocytes in vitro.Cells Tissues Organs. 2014;199(5-6):393-404. doi: 10.1159/000371342. Epub 2015 Feb 18. Cells Tissues Organs. 2014. PMID: 25720390 Free PMC article.
-
TGF-β3 reduces apoptosis in ischemia-induced adipose-derived stem cells by enhancing DNA repair.Exp Ther Med. 2018 May;15(5):4400-4408. doi: 10.3892/etm.2018.5980. Epub 2018 Mar 21. Exp Ther Med. 2018. PMID: 29725380 Free PMC article.
-
Role of STAT3-FOXO3 Signaling in the Modulation of Neuroplasticity by PD-L1-HGF-Decorated Mesenchymal Stem Cell-Derived Exosomes in a Murine Stroke Model.Adv Sci (Weinh). 2024 Sep;11(36):e2404882. doi: 10.1002/advs.202404882. Epub 2024 Jul 25. Adv Sci (Weinh). 2024. PMID: 39049677 Free PMC article.
-
Oxygen generating biomaterials at the forefront of regenerative medicine: advances in bone regeneration.Front Bioeng Biotechnol. 2024 Jan 12;12:1292171. doi: 10.3389/fbioe.2024.1292171. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38282892 Free PMC article. Review.
-
Gelatin hydrogel nonwoven fabrics of a cell culture scaffold to formulate 3-dimensional cell constructs.Regen Ther. 2021 Oct 14;18:418-429. doi: 10.1016/j.reth.2021.09.008. eCollection 2021 Dec. Regen Ther. 2021. PMID: 34722838 Free PMC article.
References
-
- Quarto R, Mastrogiacomo M, Cancedda R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–6. - PubMed
-
- Ohgushi H, Kotobuki N, Funaoka H, et al. Tissue engineered ceramic artificial joint–ex vivo osteogenic differentiation of patient mesenchymal cells on total ankle joints for treatment of osteoarthritis. Biomaterials. 2005;26:4654–61. - PubMed
-
- Kitoh H, Kitakoji T, Tsuchiya H, et al. Transplantation of marrow-derived mesenchymal stem cells and platelet-rich plasma during distraction osteogenesis–a preliminary result of three cases. Bone. 2004;35:892–8. - PubMed
-
- Vacanti CA, Bonassar LJ, Vacanti MP, et al. Replacement of an avulsed phalanx with tissue-engineered bone. N Engl J Med. 2001;344:1511–4. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

