Influence of methotrexate exposure on outcome in patients treated with MBVP chemotherapy for primary central nervous system lymphoma
- PMID: 20716237
- PMCID: PMC2949909
- DOI: 10.1111/j.1365-2125.2010.03712.x
Influence of methotrexate exposure on outcome in patients treated with MBVP chemotherapy for primary central nervous system lymphoma
Abstract
What is already known about this subject: Although treated using the same high-dose methotrexate (HD-MTX)-based multiagent chemotherapy, patients with primary central nervous system lymphoma (PCNSL) have significant differences in outcome. However, little information has been published about factors influencing outcome in PCNSL. As it is known that the pharmacokinetics of MTX vary considerably between subjects leading to different exposure in patients receiving the same dose, it is important to evaluate its role in response to chemotherapy.
What this study adds: This study is the first to evaluate the exposure-response relationship in patients treated with MBVP chemotherapy. We found that patients who were early non-responders to MBVP chemotherapy had poor survival, whatever the salvage regimen. Tumour response at early evaluation was not associated with MTX pharmacokinetics and increasing the dose would probably not improve results.
Aims: Although the standard treatment for primary central nervous system lymphoma (PCNSL) consists of three cycles of MBVP (methotrexate, BCNU, VP16, methylprednisolone) and radiotherapy, early failure of treatment may require modification of the treatment. However, our understanding of the outcome in such patients and of the factors involved in early failure of treatment is poor. In addition to known prognostic factors, we evaluated the influence of methotrexate (MTX) exposure on the response to MBVP chemotherapy in patients treated for PCNSL after the first two cycles.
Methods: We retrospectively analyzed all patients with PCNSL treated with the MBVP regimen over the previous 10 years. Clinical, personal data and known prognostic factors were studied. The parameters of MTX exposure were estimated using a population pharmacokinetic approach with NONMEM. Objective response (OR), overall survival (OS) and failure-free survival (FFS) were evaluated in all patients.
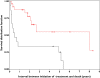
Results: Thirty-seven patients were studied. We observed lower FFS and OS (0.49 years) in patients who were not able to receive the planned treatment (group 1, n=12) than in those who received three cycles (8.04 years) (group 2, n=25). Known prognostic factors were comparable in both groups, but mean dose of MTX and mean AUC tended to be lower in patients who failed prematurely or showed no response after two cycles.
Conclusions: We found that patients who were early non-responders to MBVP chemotherapy had poor survival, without major influence of MTX exposure. It is thus probably unlikely that increasing the dose of MTX would improve outcome.
Figures
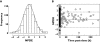
Similar articles
-
Rituximab in patients with primary CNS lymphoma (HOVON 105/ALLG NHL 24): a randomised, open-label, phase 3 intergroup study.Lancet Oncol. 2019 Feb;20(2):216-228. doi: 10.1016/S1470-2045(18)30747-2. Epub 2019 Jan 7. Lancet Oncol. 2019. PMID: 30630772 Clinical Trial.
-
High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial 20962.J Clin Oncol. 2003 Dec 15;21(24):4483-8. doi: 10.1200/JCO.2003.03.108. Epub 2003 Nov 3. J Clin Oncol. 2003. PMID: 14597741 Clinical Trial.
-
High-dose methotrexate-based chemotherapy for induction remission of newly diagnosed primary CNS lymphoma: A systematic review and meta-analysis.Medicine (Baltimore). 2025 Jan 31;104(5):e41363. doi: 10.1097/MD.0000000000041363. Medicine (Baltimore). 2025. PMID: 39889167 Free PMC article.
-
Clinical characteristics and survival outcomes of patients with primary central nervous system lymphoma treated with high-dose methotrexate-based polychemotherapy and consolidation therapies.Eur J Cancer. 2024 Dec;213:115068. doi: 10.1016/j.ejca.2024.115068. Epub 2024 Oct 13. Eur J Cancer. 2024. PMID: 39427440
-
Clinical utility and pharmacology of high-dose methotrexate in the treatment of primary CNS lymphoma.Expert Rev Neurother. 2006 May;6(5):635-52. doi: 10.1586/14737175.6.5.635. Expert Rev Neurother. 2006. PMID: 16734512 Review.
Cited by
-
Population Pharmacokinetic and Toxicity Analysis of High-Dose Methotrexate in Patients with Central Nervous System Lymphoma.Clin Pharmacokinet. 2025 Jan;64(1):79-91. doi: 10.1007/s40262-024-01452-6. Epub 2024 Dec 3. Clin Pharmacokinet. 2025. PMID: 39625585
-
Dosing algorithm to target a predefined AUC in patients with primary central nervous system lymphoma receiving high dose methotrexate.Br J Clin Pharmacol. 2012 Feb;73(2):240-7. doi: 10.1111/j.1365-2125.2011.04084.x. Br J Clin Pharmacol. 2012. PMID: 21838788 Free PMC article. Clinical Trial.
-
Population pharmacokinetics of ciclosporin in Chinese children with aplastic anemia: effects of weight, renal function and stanozolol administration.Acta Pharmacol Sin. 2013 Jul;34(7):969-75. doi: 10.1038/aps.2013.9. Epub 2013 Apr 29. Acta Pharmacol Sin. 2013. PMID: 23624757 Free PMC article.
-
Drug Exposure to Establish Pharmacokinetic-Response Relationships in Oncology.Clin Pharmacokinet. 2020 Feb;59(2):123-135. doi: 10.1007/s40262-019-00828-3. Clin Pharmacokinet. 2020. PMID: 31654368 Review.
References
-
- Bastard C, Tilly H, Lenormand B, Bigorgne C, Boulet D, Kunlin A, Monconduit M, Piguet H. Translocations involving band 3q27 and Ig gene regions in non-Hodgkin's lymphoma. Blood. 1992;79:2527–31. - PubMed
-
- Lister A, Abrey LE, Sandlund JT. Central nervous system lymphoma. Hematology Am Soc Hematol Educ Program. 2002:283–96. - PubMed
-
- DeAngelis LM, Seiferheld W, Schold SC, Fisher B, Schultz CJ. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93–10. J Clin Oncol. 2002;20:4643–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

