Reactive oxygen species and alpha,beta-unsaturated aldehydes as second messengers in signal transduction
- PMID: 20716281
- PMCID: PMC4226340
- DOI: 10.1111/j.1749-6632.2010.05551.x
Reactive oxygen species and alpha,beta-unsaturated aldehydes as second messengers in signal transduction
Abstract
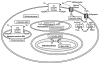
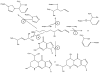
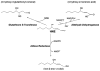
Signaling by H(2)O(2), alpha,beta-unsaturated aldehydes, such as 4-hydroxy-2-nonenal (HNE) and related chemical species, is thought to differ from signaling by other second messengers because the oxidants and other electrophiles can readily undergo nonenzymatic reactions and are therefore classified as "reactive." This brief review will describe how and when the chemistry of signaling is similar or differs from classic second messengers, such as cyclic AMP, or posttranslational signaling, such as farnesylation or ubiquitination. The chemistry of cysteine provides a common factor that underlies signaling by H(2)O(2) and HNE. Nonetheless, as H(2)O(2) and HNE are rapidly metabolized in vivo, spatial considerations are extremely important in their actions. Therefore, the locations of sources of H(2)O(2) and alpha,beta-unsaturated aldehydes, the NADPH oxidases, mitochondria, membrane lipids, and redox cycling toxicants, as well as their targets, are key factors. The activation of the JNK pathway by HNE and endogenously generated H(2)O(2) illustrates these principles.
Conflict of interest statement
The author declares no conflicts of interest.
Figures
Similar articles
-
The chemistry of cell signaling by reactive oxygen and nitrogen species and 4-hydroxynonenal.Arch Biochem Biophys. 2008 Sep 15;477(2):183-95. doi: 10.1016/j.abb.2008.06.011. Epub 2008 Jun 24. Arch Biochem Biophys. 2008. PMID: 18602883 Free PMC article. Review.
-
Signaling functions of reactive oxygen species.Biochemistry. 2010 Feb 9;49(5):835-42. doi: 10.1021/bi9020378. Biochemistry. 2010. PMID: 20050630 Free PMC article. Review.
-
Reactive aldehydes--second messengers of free radicals in diabetes mellitus.Free Radic Res. 2013 Aug;47 Suppl 1:39-48. doi: 10.3109/10715762.2013.789136. Epub 2013 Apr 25. Free Radic Res. 2013. PMID: 23521622 Review.
-
Endogenous 4-hydroxy-2-nonenal in microalga Chlorella kessleri acts as a bioactive indicator of pollution with common herbicides and growth regulating factor of hormesis.Aquat Toxicol. 2011 Oct;105(3-4):552-8. doi: 10.1016/j.aquatox.2011.08.007. Epub 2011 Aug 22. Aquat Toxicol. 2011. PMID: 21937009
-
4-hydroxynonenal contributes to NGF withdrawal-induced neuronal apoptosis.J Neurochem. 2003 May;85(4):999-1005. doi: 10.1046/j.1471-4159.2003.01749.x. J Neurochem. 2003. PMID: 12716431
Cited by
-
Fructation in vivo: detrimental and protective effects of fructose.Biomed Res Int. 2013;2013:343914. doi: 10.1155/2013/343914. Epub 2013 Jul 24. Biomed Res Int. 2013. PMID: 23984346 Free PMC article. Review.
-
An overview of mechanisms of redox signaling.J Mol Cell Cardiol. 2014 Aug;73:2-9. doi: 10.1016/j.yjmcc.2014.01.018. Epub 2014 Feb 8. J Mol Cell Cardiol. 2014. PMID: 24512843 Free PMC article. Review.
-
Differential metabolism of 4-hydroxynonenal in liver, lung and brain of mice and rats.Toxicol Appl Pharmacol. 2014 Aug 15;279(1):43-52. doi: 10.1016/j.taap.2014.04.026. Epub 2014 May 14. Toxicol Appl Pharmacol. 2014. PMID: 24832492 Free PMC article.
-
Mitochondrial maintenance failure in aging and role of sexual dimorphism.Arch Biochem Biophys. 2015 Jun 15;576:17-31. doi: 10.1016/j.abb.2014.10.008. Epub 2014 Oct 25. Arch Biochem Biophys. 2015. PMID: 25447815 Free PMC article. Review.
-
Fatty acid amide hydrolase is a key regulator of endocannabinoid-induced myocardial tissue injury.Free Radic Biol Med. 2011 Jan 1;50(1):179-95. doi: 10.1016/j.freeradbiomed.2010.11.002. Epub 2010 Nov 9. Free Radic Biol Med. 2011. PMID: 21070851 Free PMC article.
References
-
- Cheng G, et al. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. - PubMed
-
- Lambeth JD. Nox/Duox family of nicotinamide adenine dinucleotide (phosphate) oxidases. Curr Opin Hematol. 2002;9:11–17. - PubMed
-
- Loschen G, et al. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974;42:68–72. - PubMed
-
- Forman HJ, Kennedy JA. Role of superoxide radical in mitochondrial dehydrogenase reactions. Biochem Biophys Res Commun. 1974;60:1044–1050. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

