Mechanisms of oxidant generation by catalase
- PMID: 20716293
- PMCID: PMC4610122
- DOI: 10.1111/j.1749-6632.2010.05603.x
Mechanisms of oxidant generation by catalase
Abstract
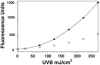
The enzyme catalase converts solar radiation into reactive oxidant species (ROS). In this study, we report that several bacterial catalases (hydroperoxidases, HP), including Escherichia coli HP-I and HP-II also generate reactive oxidants in response to ultraviolet B light (UVB). HP-I and HP-II are identical except for the presence of NADPH. We found that only one of the catalases, HPI, produces oxidants in response to UVB light, indicating a potential role for the nucleotide in ROS production. This prompts us to speculate that NADPH may act as a cofactor regulating ROS generation by mammalian catalases. Structural analysis of the NADPH domains of several mammalian catalases revealed that the nucleotide is bound in a constrained conformation and that UVB irradiation induces NADPH oxidation and positional changes. Biochemical and kinetic analysis indicate that ROS formation by the enzyme is enhanced by oxidation of the cofactor. Conformational changes following absorption of UVB light by catalase NADPH have the potential to facilitate ROS production by the enzyme.
Figures


References
-
- Jamieson D, Chance B, Cadenas E, Boveris A. The relation of free radical production to hyperoxia. Annu. Rev. Physiol. 1986;48:703–719. - PubMed
-
- Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. - PubMed
-
- Heck DE, Kagan VE, Shvedova AA, Laskin JD. An epigrammatic (abridged) recounting of the myriad tales of astonishing deeds and dire consequences pertaining to nitric oxide and reactive oxygen species in mitochondria with an ancillary missive concerning the origins of apoptosis. Toxicology. 2005;208:259–271. - PubMed
-
- Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009;47:333–343. - PubMed
-
- Heck DE, Vetrano AM, Mariano TM, Laskin JD. UVB light stimulates production of reactive oxygen species: unexpected role for catalase. J. Biol. Chem. 2003;278:22432–22436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

