Systematic Review of topotecan (Hycamtin) in relapsed small cell lung cancer
- PMID: 20716361
- PMCID: PMC2931489
- DOI: 10.1186/1471-2407-10-436
Systematic Review of topotecan (Hycamtin) in relapsed small cell lung cancer
Abstract
Background: To undertake a systematic review of the available data for oral and intravenous topotecan in adults with relapsed small cell lung cancer (SCLC) for whom re-treatment with the first line regimen is not considered appropriate.
Methods: We searched six databases from 1980 up to March 2009 for relevant trials regardless of language or publication status. Relevant studies included any randomised trial of any chemotherapeutic treatment against any comparator in this licensed indication. Where possible we used opposite quantitative methods. Where meta-analysis was considered unsuitable for some or all of the data, we employed a narrative synthesis method. For indirect comparisons we used the method of Bucher et al., where available data allowed it, otherwise we used narrative descriptions.
Results: Seven unique studies met the inclusion criteria, four of which could be used in our analyses. These included one study comparing oral topotecan plus best supportive care (BSC) to BSC alone, one study comparing intravenous topotecan to cyclophosphamide, adriamycin and vincristine (CAV), and two studies comparing oral topotecan with intravenous topotecan. All four studies appear to be well conducted and with low risk of bias. Oral topotecan plus BSC has advantages over BSC alone in terms of survival (hazard ratio = 0.61; 95% CI, 0.43 to 0.87) and quality of life (EQ-5 D difference: 0.15; 95% CI, 0.05 to 0.25). Intravenous topotecan was at least as effective as CAV in the treatment of patients with recurrent small-cell lung cancer and resulted in improved quality-of-life with respect to several symptoms. CAV was associated with significantly less grade 4 thrombocytopenia compared with IV topotecan (risk ratio = 5.83; 95% CI, 2.35 to 14.42). Survival (hazard ratio = 0.98; 95% CI, 0.77 to 1.25) and response (pooled risk ratio = 1.04; 95% CI, 0.58 to 1.85) data were similar for the oral and IV topotecan groups. Symptom control was also very similar between the trials and between the oral and IV groups. Toxicity data showed a significant difference in favour of oral topotecan for neutropenia (pooled risk ratio = 0.65; 95% CI, 0.47 to 0.89). Indirect evidence showed that oral topotecan was at least as good as or better than CAV on all outcomes (survival, response rates, toxicities, and symptoms) that allowed indirect comparisons, with the only exception being grade four thrombocytopenia which occurred less often on CAV treatment.
Conclusions: Concerning topotecan both the oral and intravenous options have similar efficacy, and patient preference may be a decisive factor if the choice would be between the two formulations. The best trial evidence for decision making, because it was tested versus best supportive care, exists for oral topotecan. Indirectly, because we have two head-to-head comparisons of oral versus intravenous topotecan, and one comparison of intravenous topotecan versus CAV in similar patients as in the trial against best supportive care, one might infer that IV topotecan and CAV could also be superior to best supportive care, and that oral topotecan has similar effects to CAV with possibly better symptom control. From the evidence discussed above, it is evident that oral topotecan has similar efficacy to IV topotecan (direct comparison) and CAV (indirect comparison). There is no further evidence base of direct or possible indirect comparisons for other comparators than CAV of either oral or IV topotecan.
Figures

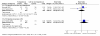
References
-
- Office for National Statistics. Cancer statistics registrations. Registrations of cancer diagnosed in 2005, England. 2008. Newport, HMSO; Series MB1 no. 36.
-
- Welsh Cancer Intelligence & Surveillance Unit. Cancer Incidence in Wales 2002-2006. WCISU; SA8/01. 2008. http://www.wales.nhs.uk/sites3/Documents/242/incpub2006%5F31Jan08.pdf Cited 4-6-2009.
-
- Hyde L, Yee J, Wilson R, Patno ME. Cell type and the natural history of lung cancer. JAMA. 1965;193:52–54. - PubMed
-
- Lassen U, Osterlind K, Hansen M, Dombernowsky P, Bergman B, Hansen HH. Longterm survival in small-cell lung cancer: posttreatment characteristics in patients surviving 5 to 18+ years - an analysis of 1,714 consecutive patients. J Clin Oncol. 1995;13(5):1215–1220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

