Diversity and strength of internal outward-oriented promoters in group IIC-attC introns
- PMID: 20716518
- PMCID: PMC3001079
- DOI: 10.1093/nar/gkq709
Diversity and strength of internal outward-oriented promoters in group IIC-attC introns
Abstract
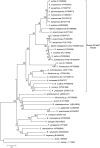
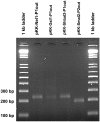
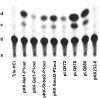
Integrons are genetic elements that incorporate mobile gene cassettes by site-specific recombination and express them as an operon from a promoter (Pc) located upstream of the cassette insertion site. Most gene cassettes found in integrons contain only one gene followed by an attC recombination site. We have recently shown that a specific lineage of group IIC introns, named group IIC-attC introns, inserts into the bottom strand sequence of attC sites. Here, we show that S.ma.I2, a group IIC-attC intron inserted in an integron cassette array of Serratia marcescens, impedes transcription from Pc while allowing expression of the following antibiotic resistance cassette using an internal outward-oriented promoter (P(out)). Bioinformatic analyses indicate that one or two putative P(out), which have sequence similarities with the Escherichia coli consensus promoters, are conserved in most group IIC-attC intron sequences. We show that P(out) with different versions of the -35 and -10 sequences are functionally active in expressing a promoterless chloramphenicol acetyltransferase (cat) reporter gene in E. coli. P(out) in group IIC-attC introns may therefore play a role in the expression of one or more gene cassettes whose transcription from Pc would otherwise be impeded by insertion of the intron.
Figures



Similar articles
-
Potential role of group IIC-attC introns in integron cassette formation.J Bacteriol. 2009 Oct;191(19):6040-51. doi: 10.1128/JB.00674-09. Epub 2009 Jul 24. J Bacteriol. 2009. PMID: 19633079 Free PMC article.
-
Group IIC intron mobility into attC sites involves a bulged DNA stem-loop motif.RNA. 2009 Aug;15(8):1543-53. doi: 10.1261/rna.1649309. Epub 2009 Jun 9. RNA. 2009. PMID: 19509303 Free PMC article.
-
The S.ma.I2 class C group II intron inserts at integron attC sites.Microbiology (Reading). 2008 May;154(Pt 5):1341-1353. doi: 10.1099/mic.0.2007/016360-0. Microbiology (Reading). 2008. PMID: 18451043
-
Integrons and gene cassettes: hotspots of diversity in bacterial genomes.Ann N Y Acad Sci. 2012 Sep;1267:71-8. doi: 10.1111/j.1749-6632.2012.06588.x. Ann N Y Acad Sci. 2012. PMID: 22954219 Review.
-
Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination.Mol Microbiol. 1995 Feb;15(4):593-600. doi: 10.1111/j.1365-2958.1995.tb02368.x. Mol Microbiol. 1995. PMID: 7783631 Review.
Cited by
-
Evolution of group II introns.Mob DNA. 2015 Apr 1;6:7. doi: 10.1186/s13100-015-0037-5. eCollection 2015. Mob DNA. 2015. PMID: 25960782 Free PMC article.
-
RmtF, a new member of the aminoglycoside resistance 16S rRNA N7 G1405 methyltransferase family.Antimicrob Agents Chemother. 2012 Jul;56(7):3960-2. doi: 10.1128/AAC.00660-12. Epub 2012 Apr 30. Antimicrob Agents Chemother. 2012. PMID: 22547620 Free PMC article.
-
Integron Functionality and Genome Innovation: An Update on the Subtle and Smart Strategy of Integrase and Gene Cassette Expression Regulation.Microorganisms. 2022 Jan 20;10(2):224. doi: 10.3390/microorganisms10020224. Microorganisms. 2022. PMID: 35208680 Free PMC article. Review.
-
The influence of the accessory genome on bacterial pathogen evolution.Mob Genet Elements. 2011 May;1(1):55-65. doi: 10.4161/mge.1.1.16432. Mob Genet Elements. 2011. PMID: 22016845 Free PMC article.
-
The association of group IIB intron with integrons in hypersaline environments.Mob DNA. 2021 Mar 1;12(1):8. doi: 10.1186/s13100-021-00234-2. Mob DNA. 2021. PMID: 33648565 Free PMC article.
References
-
- Lévesque C, Brassard S, Lapointe J, Roy PH. Diversity and relative strength of tandem promoters for the antibiotic-resistance genes of several integrons. Gene. 1994;142:49–54. - PubMed
-
- Stokes HW, Hall RM. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol. Microbiol. 1989;3:1669–1683. - PubMed
-
- Hall RM, Brookes DE, Stokes HW. Site-specific insertion of genes into integrons: role of the 59-base element and determination of the recombination cross-over point. Mol. Microbiol. 1991;5:1941–1959. - PubMed
-
- Boucher Y, Labbate M, Koenig JE, Stokes HW. Integrons: mobilizable platforms that promote genetic diversity in bacteria. Trends Microbiol. 2007;15:301–309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

