A major fraction of glycosphingolipids in model and cellular cholesterol-containing membranes is undetectable by their binding proteins
- PMID: 20716521
- PMCID: PMC2975227
- DOI: 10.1074/jbc.M110.110189
A major fraction of glycosphingolipids in model and cellular cholesterol-containing membranes is undetectable by their binding proteins
Abstract
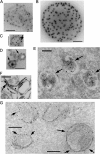
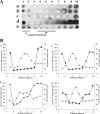
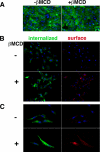
Glycosphingolipids (GSLs) accumulate in cholesterol-enriched cell membrane domains and provide receptors for protein ligands. Lipid-based "aglycone" interactions can influence GSL carbohydrate epitope presentation. To evaluate this relationship, Verotoxin binding its receptor GSL, globotriaosyl ceramide (Gb(3)), was analyzed in simple GSL/cholesterol, detergent-resistant membrane vesicles by equilibrium density gradient centrifugation. Vesicles separated into two Gb(3/)cholesterol-containing populations. The lighter, minor fraction (<5% total GSL), bound VT1, VT2, IgG/IgM mAb anti-Gb(3), HIVgp120 or Bandeiraea simplicifolia lectin. Only IgM anti-Gb(3), more tolerant of carbohydrate modification, bound both vesicle fractions. Post-embedding cryo-immuno-EM confirmed these results. This appears to be a general GSL-cholesterol property, because similar receptor-inactive vesicles were separated for other GSL-protein ligand systems; cholera toxin (CTx)-GM1, HIVgp120-galactosyl ceramide/sulfatide. Inclusion of galactosyl or glucosyl ceramide (GalCer and GlcCer) rendered VT1-unreactive Gb(3)/cholesterol vesicles, VT1-reactive. We found GalCer and GlcCer bind Gb(3), suggesting GSL-GSL interaction can counter cholesterol masking of Gb(3). The similar separation of Vero cell membrane-derived vesicles into minor "binding," and major "non-binding" fractions when probed with VT1, CTx, or anti-SSEA4 (a human GSL stem cell marker), demonstrates potential physiological relevance. Cell membrane GSL masking was cholesterol- and actin-dependent. Cholesterol depletion of Vero and HeLa cells enabled differential VT1B subunit labeling of "available" and "cholesterol-masked" plasma membrane Gb(3) pools by fluorescence microscopy. Thus, the model GSL/cholesterol vesicle studies predicted two distinct membrane GSL formats, which were demonstrated within the plasma membrane of cultured cells. Cholesterol masking of most cell membrane GSLs may impinge many GSL receptor functions.
Figures






Similar articles
-
Structure-dependent pseudoreceptor intracellular traffic of adamantyl globotriaosyl ceramide mimics.J Biol Chem. 2012 May 11;287(20):16073-87. doi: 10.1074/jbc.M111.318196. Epub 2012 Mar 14. J Biol Chem. 2012. PMID: 22418442 Free PMC article.
-
Fatty acid-dependent globotriaosyl ceramide receptor function in detergent resistant model membranes.J Lipid Res. 2009 Sep;50(9):1744-55. doi: 10.1194/jlr.M800385-JLR200. Epub 2008 Aug 20. J Lipid Res. 2009. PMID: 18716315 Free PMC article.
-
Differential intracellular transport and binding of verotoxin 1 and verotoxin 2 to globotriaosylceramide-containing lipid assemblies.J Cell Physiol. 2008 Sep;216(3):750-63. doi: 10.1002/jcp.21456. J Cell Physiol. 2008. PMID: 18446787
-
New aspects of the regulation of glycosphingolipid receptor function.Chem Phys Lipids. 2010 Jan;163(1):27-35. doi: 10.1016/j.chemphyslip.2009.09.001. Chem Phys Lipids. 2010. PMID: 19781539 Review.
-
Globotriaosyl ceramide receptor function - where membrane structure and pathology intersect.FEBS Lett. 2010 May 3;584(9):1879-86. doi: 10.1016/j.febslet.2009.11.089. Epub 2009 Dec 2. FEBS Lett. 2010. PMID: 19948172 Review.
Cited by
-
Mechanisms underlying the confined diffusion of cholera toxin B-subunit in intact cell membranes.PLoS One. 2012;7(4):e34923. doi: 10.1371/journal.pone.0034923. Epub 2012 Apr 12. PLoS One. 2012. PMID: 22511973 Free PMC article.
-
Structure-dependent pseudoreceptor intracellular traffic of adamantyl globotriaosyl ceramide mimics.J Biol Chem. 2012 May 11;287(20):16073-87. doi: 10.1074/jbc.M111.318196. Epub 2012 Mar 14. J Biol Chem. 2012. PMID: 22418442 Free PMC article.
-
Hetero-Multivalency of Pseudomonas aeruginosa Lectin LecA Binding to Model Membranes.Sci Rep. 2018 May 30;8(1):8419. doi: 10.1038/s41598-018-26643-7. Sci Rep. 2018. PMID: 29849092 Free PMC article.
-
Cell density-induced changes in lipid composition and intracellular trafficking.Cell Mol Life Sci. 2014 Mar;71(6):1097-116. doi: 10.1007/s00018-013-1441-y. Epub 2013 Aug 7. Cell Mol Life Sci. 2014. PMID: 23921715 Free PMC article.
-
Glycolipid binding preferences of Shiga toxin variants.PLoS One. 2014 Jul 1;9(7):e101173. doi: 10.1371/journal.pone.0101173. eCollection 2014. PLoS One. 2014. PMID: 24983355 Free PMC article.
References
-
- Lingwood C. A. (1996) Glycoconj. J. 13, 495–503 - PubMed
-
- Ackerman G. A., Wolken K. W., Gelder F. B. (1980) J. Histochem. Cytochem. 28, 1334–1342 - PubMed
-
- Hakomori S. (1981) Annu. Rev. Biochem. 50, 733–764 - PubMed
-
- Nakakuma H., Horikawa K., Kawaguchi T., Hidaka M., Nagakura S., Hirai S., Kageshita T., Ono T., Kagimoto T., Iwamori M., et al. (1992) Jpn. J. Clin. Oncol. 22, 308–312 - PubMed
-
- Kannagi R., Stroup R., Cochran N. A., Urdal D. L., Young W. W., Jr., Hakomori S. I. (1983) Cancer Res. 43, 4997–5005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

