Metabolic depression and increased reactive oxygen species production by isolated mitochondria at moderately lower temperatures
- PMID: 20716522
- PMCID: PMC2952254
- DOI: 10.1074/jbc.M110.155432
Metabolic depression and increased reactive oxygen species production by isolated mitochondria at moderately lower temperatures
Abstract
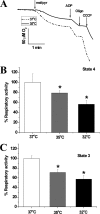
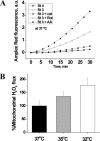
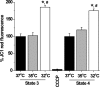
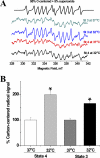
Temperature (T) reduction increases lifespan, but the mechanisms are not understood. Because reactive oxygen species (ROS) contribute to aging, we hypothesized that lowering T might decrease mitochondrial ROS production. We measured respiratory response and ROS production in isolated mitochondria at 32, 35, and 37 °C. Lowering T decreased the rates of resting (state 4) and phosphorylating (state 3) respiration phases. Surprisingly, this respiratory slowdown was associated with an increase of ROS production and hydrogen peroxide release and with elevation of the mitochondrial membrane potential, ΔΨ(m). We also found that at lower T mitochondria produced more carbon-centered lipid radicals, a species known to activate uncoupling proteins. These data indicate that reduced mitochondrial ROS production is not one of the mechanisms mediating lifespan extension at lower T. They suggest instead that increased ROS leakage may mediate mitochondrial responses to hypothermia.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

