Peroxisome proliferator-activated receptor {gamma} coactivator 1{alpha} (PGC-1{alpha}) promotes skeletal muscle lipid refueling in vivo by activating de novo lipogenesis and the pentose phosphate pathway
- PMID: 20716531
- PMCID: PMC2963391
- DOI: 10.1074/jbc.M110.145995
Peroxisome proliferator-activated receptor {gamma} coactivator 1{alpha} (PGC-1{alpha}) promotes skeletal muscle lipid refueling in vivo by activating de novo lipogenesis and the pentose phosphate pathway
Abstract
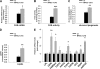
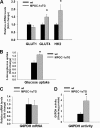
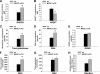
Exercise induces a pleiotropic adaptive response in skeletal muscle, largely through peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α). PGC-1α enhances lipid oxidation and thereby provides energy for sustained muscle contraction. Its potential implication in promoting muscle refueling remains unresolved, however. Here, we investigated a possible role of elevated PGC-1α levels in skeletal muscle lipogenesis in vivo and the molecular mechanisms that underlie PGC-1α-mediated de novo lipogenesis. To this end, we studied transgenic mice with physiological overexpression of PGC-1α and human muscle biopsies pre- and post-exercise. We demonstrate that PGC-1α enhances lipogenesis in skeletal muscle through liver X receptor α-dependent activation of the fatty acid synthase (FAS) promoter and by increasing FAS activity. Using chromatin immunoprecipitation, we establish a direct interaction between PGC-1α and the liver X receptor-responsive element in the FAS promoter. Moreover, we show for the first time that increased glucose uptake and activation of the pentose phosphate pathway provide substrates for RNA synthesis and cofactors for de novo lipogenesis. Similarly, we observed increased lipogenesis and lipid levels in human muscle biopsies that were obtained post-exercise. Our findings suggest that PGC-1α coordinates lipogenesis, intramyocellular lipid accumulation, and substrate oxidation in exercised skeletal muscle in vivo.
Figures
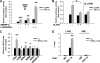

Similar articles
-
Striated muscle activator of Rho signalling (STARS) is a PGC-1α/oestrogen-related receptor-α target gene and is upregulated in human skeletal muscle after endurance exercise.J Physiol. 2011 Apr 15;589(Pt 8):2027-39. doi: 10.1113/jphysiol.2011.205468. Epub 2011 Feb 21. J Physiol. 2011. PMID: 21486805 Free PMC article.
-
Increased levels of peroxisome proliferator-activated receptor gamma, coactivator 1 alpha (PGC-1alpha) improve lipid utilisation, insulin signalling and glucose transport in skeletal muscle of lean and insulin-resistant obese Zucker rats.Diabetologia. 2010 Sep;53(9):2008-19. doi: 10.1007/s00125-010-1773-1. Epub 2010 May 20. Diabetologia. 2010. PMID: 20490453
-
PGC-1α induces mitochondrial and myokine transcriptional programs and lipid droplet and glycogen accumulation in cultured human skeletal muscle cells.PLoS One. 2012;7(1):e29985. doi: 10.1371/journal.pone.0029985. Epub 2012 Jan 17. PLoS One. 2012. PMID: 22272266 Free PMC article.
-
PGC-1alpha-induced improvements in skeletal muscle metabolism and insulin sensitivity.Appl Physiol Nutr Metab. 2009 Jun;34(3):307-14. doi: 10.1139/H09-008. Appl Physiol Nutr Metab. 2009. PMID: 19448691 Review.
-
PGC-1alpha-mediated adaptations in skeletal muscle.Pflugers Arch. 2010 Jun;460(1):153-62. doi: 10.1007/s00424-010-0834-0. Epub 2010 Apr 19. Pflugers Arch. 2010. PMID: 20401754 Review.
Cited by
-
Dose- and time-dependent alterations in lipid metabolism after pharmacological PGC-1α activation in L6 myotubes.J Cell Physiol. 2019 Jul;234(7):11923-11941. doi: 10.1002/jcp.27872. Epub 2018 Dec 6. J Cell Physiol. 2019. PMID: 30523639 Free PMC article.
-
Hypoxia Induces Saturated Fatty Acids Accumulation and Reduces Unsaturated Fatty Acids Independently of Reverse Tricarboxylic Acid Cycle in L6 Myotubes.Front Endocrinol (Lausanne). 2022 Mar 11;13:663625. doi: 10.3389/fendo.2022.663625. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35360057 Free PMC article.
-
PGC1α promotes tumor growth by inducing gene expression programs supporting lipogenesis.Cancer Res. 2011 Nov 1;71(21):6888-98. doi: 10.1158/0008-5472.CAN-11-1011. Epub 2011 Sep 13. Cancer Res. 2011. PMID: 21914785 Free PMC article.
-
Multiparity leads to obesity and inflammation in mothers and obesity in male offspring.Am J Physiol Endocrinol Metab. 2012 Feb 15;302(4):E449-57. doi: 10.1152/ajpendo.00487.2011. Epub 2011 Nov 29. Am J Physiol Endocrinol Metab. 2012. PMID: 22127227 Free PMC article.
-
The impact of bed rest on human skeletal muscle metabolism.Cell Rep Med. 2024 Jan 16;5(1):101372. doi: 10.1016/j.xcrm.2023.101372. Cell Rep Med. 2024. PMID: 38232697 Free PMC article.
References
-
- Booth F. W., Laye M. J., Lees S. J., Rector R. S., Thyfault J. P. (2008) Eur. J. Appl. Physiol. 102, 381–390 - PubMed
-
- Flück M., Hoppeler H. (2003) Rev. Physiol. Biochem. Pharmacol. 146, 159–216 - PubMed
-
- Handschin C., Spiegelman B. M. (2006) Endocr. Rev. 27, 728–735 - PubMed
-
- Lin J., Handschin C., Spiegelman B. M. (2005) Cell Metab. 1, 361–370 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

