Frequent deregulations in the hedgehog signaling network and cross-talks with the epidermal growth factor receptor pathway involved in cancer progression and targeted therapies
- PMID: 20716670
- PMCID: PMC2964899
- DOI: 10.1124/pr.109.002329
Frequent deregulations in the hedgehog signaling network and cross-talks with the epidermal growth factor receptor pathway involved in cancer progression and targeted therapies
Abstract
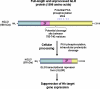
The hedgehog (Hh)/glioma-associated oncogene (GLI) signaling network is among the most important and fascinating signal transduction systems that provide critical functions in the regulation of many developmental and physiological processes. The coordinated spatiotemporal interplay of the Hh ligands and other growth factors is necessary for the stringent control of the behavior of diverse types of tissue-resident stem/progenitor cells and their progenies. The activation of the Hh cascade might promote the tissue regeneration and repair after severe injury in numerous organs, insulin production in pancreatic beta-cells, and neovascularization. Consequently, the stimulation of the Hh pathway constitutes a potential therapeutic strategy to treat diverse human disorders, including severe tissue injuries; diabetes mellitus; and brain, skin, and cardiovascular disorders. In counterbalance, a deregulation of the Hh signaling network might lead to major tissular disorders and the development of a wide variety of aggressive and metastatic cancers. The target gene products induced through the persistent Hh activation can contribute to the self-renewal, survival, migration, and metastasis of cancer stem/progenitor cells and their progenies. Moreover, the pivotal role mediated through the Hh/GLI cascade during cancer progression also implicates the cooperation with other oncogenic products, such as mutated K-RAS and complex cross-talk with different growth factor pathways, including tyrosine kinase receptors, such as epidermal growth factor receptor (EGFR), Wnt/beta-catenin, and transforming growth factor-beta (TGF-beta)/TGF-beta receptors. Therefore, the molecular targeting of distinct deregulated gene products, including Hh and EGFR signaling components and other signaling elements that are frequently deregulated in highly tumorigenic cancer-initiating cells and their progenies, might constitute a potential therapeutic strategy to eradicate the total cancer cell mass. Of clinical interest is that these multitargeted approaches offer great promise as adjuvant treatments for improving the current antihormonal therapies, radiotherapies, and/or chemotherapies against locally advanced and metastatic cancers, thereby preventing disease relapse and the death of patients with cancer.
Figures





References
-
- Ahn S, Joyner AL. (2005) In vivo analysis of quiescent adult neural stem cells responding to sonic hedgehog. Nature 437:894–897 - PubMed
-
- Alvarez-Medina R, Le Dreau G, Ros M, Martí E. (2009) Hedgehog activation is required upstream of Wnt signalling to control neural progenitor proliferation. Development 136:3301–3309 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

