Novel, unifying mechanism for mescaline in the central nervous system: electrochemistry, catechol redox metabolite, receptor, cell signaling and structure activity relationships
- PMID: 20716904
- PMCID: PMC2763256
- DOI: 10.4161/oxim.2.4.9380
Novel, unifying mechanism for mescaline in the central nervous system: electrochemistry, catechol redox metabolite, receptor, cell signaling and structure activity relationships
Abstract
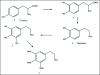
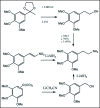
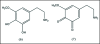
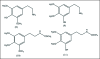
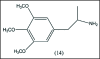
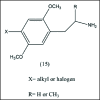
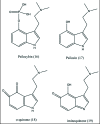
A unifying mechanism for abused drugs has been proposed previously from the standpoint of electron transfer. Mescaline can be accommodated within the theoretical framework based on redox cycling by the catechol metabolite with its quinone counterpart. Electron transfer may play a role in electrical effects involving the nervous system in the brain. This approach is in accord with structure activity relationships involving mescaline, abused drugs, catecholamines, and etoposide. Inefficient demethylation is in keeping with the various drug properties, such as requirement for high dosage and slow acting. There is a discussion of receptor binding, electrical effects, cell signaling and other modes of action. Mescaline is a nonselective, seretonin receptor agonist. 5-HTP receptors are involved in the stimulus properties. Research addresses the aspect of stereochemical requirements. Receptor binding may involve the proposed quinone metabolite and/or the amino sidechain via protonation. Electroencephalographic studies were performed on the effects of mescaline on men. Spikes are elicited by stimulation of a cortical area. The potentials likely originate in nonsynaptic dendritic membranes. Receptor-mediated signaling pathways were examined which affect mescaline behavior. The hallucinogen belongs to the class of 2AR agonists which regulate pathways in cortical neurons. The research identifies neural and signaling mechanisms responsible for the biological effects. Recently, another hallucinogen, psilocybin, has been included within the unifying mechanistic framework. This mushroom constituent is hydrolyzed to the phenol psilocin, also active, which is subsequently oxidized to an ET o-quinone or iminoquinone.
Figures
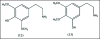
References
-
- Mc Laughlin JL. Peyote: an introduction. Lloydia. 1973;36:1–8. - PubMed
-
- Paul AG. Biosynthesis of the peyote alkaloids. Lloydia. 1973;36:36–45. - PubMed
-
- Lundstöm J, Agurell S. A complete biosynthetic sequence from tyrosine to mescaline. Tetrahedron Lett. 1969:3371–3374. - PubMed
-
- Kapadia GJ, Vaishnav YN, Fayez MBE. Peyote alkaloids IX: Identification and synthesis of demethylmescaline, a plausible intermediate in the biosynthesis of the cactus alkaloids. J Pharmaceut Sci. 1969;58:1157–1159. - PubMed
-
- Soderquist JA, Kock I, Estrella ME. Reductive cleavage of acetals and ketals with 9-borabicyclo[3.3.1]nonane. Org Proc Res Develop. 2006;10:1076–1079.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

