Acacia Senegal gum exudate offers protection against cyclophosphamide-induced urinary bladder cytotoxicity
- PMID: 20716906
- PMCID: PMC2763258
- DOI: 10.4161/oxim.2.4.8878
Acacia Senegal gum exudate offers protection against cyclophosphamide-induced urinary bladder cytotoxicity
Abstract
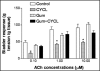
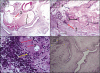
Cylophosphamide (CYCL) is a strong anticancer and immunosuppressive agent but its urotoxicity presents one of the major toxic effects that limit its wide usage particularly in high dose regimens. Therefore, this study aimed to investigate Acacia Senegal gum exudate ,Gum Arabic (GA), for its possible role as a natural, nontoxic agent against CYCL-induced urotoxicity. Male Swiss albino rats were exposed to CYCL (150 mg/kg BW, once i.p) with or without GA oral supplementation (7.5 g/kg/day for 6 days) through drinking water. Glutathione (GSH), Malondialdehyde (MDA) and Nitric oxide (NO) bladder contents were assessed. Responsiveness of the bladder rings to acetylcholine (ACh) in vitro, microscopic and macroscopic features are also investigated. CYCL produced pronounced harmful effects on bladder urothelial lining with significant increases in (MDA) and NO levels in the tissue homogenates. Bladder-GSH content is dropped by over 60% following CYCL injection. Bladder contractility, as measured by its responsiveness to ACh, recorded a marked reduction. The isolated bladders exhibited such macroscopic changes as severe edema, inflammation and extravasation. The bladder weight increased as well. Histological changes were evident in the form of severe congestion, petechial hemorrhage and chronic inflammatory reaction in the lamina propria accompanied with desquamated epithelia. GA, a potential protective agent, produced an almost complete reversal of NO induction, lipid peroxidation or cellular GSH bladder contents in the GA+CYCL-treated group. Likewise, bladder inflammation and edema were reduced. Bladder rings showed a remarkable recovery in their responsiveness to ACh. Bladder histological examination showed a near normal configuration and structural integrity, with a significant reduction in inflammation and disappearance of focal erosions. These remarkable effects of GA may be attributed to its ability to neutralize acrolein, the reactive metabolite of CYCL and/or the resultant reactive oxygen metabolites, through a scavenging action. GA may limit the cascading events of CYCL -induced damage, initiating a cytoprotective effect leading to structural and functional recovery of the bladder tissues.
Figures




Similar articles
-
Protective effect of taurine against cyclophosphamide-induced urinary bladder toxicity in rats.Clin Exp Pharmacol Physiol. 2005 Mar;32(3):167-72. doi: 10.1111/j.1440-1681.2004.04129.x. Clin Exp Pharmacol Physiol. 2005. PMID: 15743398
-
Protective effect of seleno-L-methionine on cyclophosphamide-induced urinary bladder toxicity in rats.Biol Trace Elem Res. 2010 Apr;134(1):98-108. doi: 10.1007/s12011-009-8458-y. Epub 2009 Jul 24. Biol Trace Elem Res. 2010. PMID: 19629405
-
Protective effect of S-allylcysteine against cyclophosphamide-induced bladder hemorrhagic cystitis in mice.Food Chem Toxicol. 2008 Nov;46(11):3368-74. doi: 10.1016/j.fct.2008.08.011. Epub 2008 Aug 22. Food Chem Toxicol. 2008. PMID: 18786597
-
Bladder urotoxicity pathophysiology induced by the oxazaphosphorine alkylating agents and its chemoprevention .Postepy Hig Med Dosw (Online). 2012 Sep 10;66:592-602. doi: 10.5604/17322693.1009703. Postepy Hig Med Dosw (Online). 2012. PMID: 23001201 Review.
-
Gum acacia dietary fiber: Significance in immunomodulation, inflammatory diseases, and cancer.Phytother Res. 2024 Mar;38(3):1509-1521. doi: 10.1002/ptr.8125. Epub 2024 Jan 25. Phytother Res. 2024. PMID: 38272848 Review.
Cited by
-
Effect of gum arabic on oxidative stress and inflammation in adenine-induced chronic renal failure in rats.PLoS One. 2013;8(2):e55242. doi: 10.1371/journal.pone.0055242. Epub 2013 Feb 1. PLoS One. 2013. PMID: 23383316 Free PMC article.
-
Protective effect of alogliptin against cyclophosphamide-induced lung toxicity in rats: Impact on PI3K/Akt/FoxO1 pathway and downstream inflammatory cascades.Cell Tissue Res. 2022 May;388(2):417-438. doi: 10.1007/s00441-022-03593-1. Epub 2022 Feb 2. Cell Tissue Res. 2022. PMID: 35107620 Free PMC article.
-
Uroprotective effect of ambroxol in cyclophosphamide-induced cystitis in mice.Int Urol Nephrol. 2019 May;51(5):803-810. doi: 10.1007/s11255-019-02128-y. Epub 2019 Mar 20. Int Urol Nephrol. 2019. PMID: 30895504
-
Cyclophosphamide induced stomach and duodenal lesions as a NO-system disturbance in rats: L-NAME, L-arginine, stable gastric pentadecapeptide BPC 157.Inflammopharmacology. 2017 Apr;25(2):255-264. doi: 10.1007/s10787-017-0330-7. Epub 2017 Mar 2. Inflammopharmacology. 2017. PMID: 28255738
-
Ameliorative Effect of Gum Acacia on Hookah Smoke-Induced Testicular Impairment in Mice.Biomolecules. 2020 May 13;10(5):762. doi: 10.3390/biom10050762. Biomolecules. 2020. PMID: 32414135 Free PMC article.
References
-
- Moore MJ. Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet. 1991;20:194–208. - PubMed
-
- Hengstler JG, Hengst A, Fuchs J, Tanner B, Phol J, Oesch F. Induction of DNA crosslinks and DNA strand lesions by cyclophosphamide after activation by cytochrome P450 2B1. Mutat Res. 1997;373:215–223. - PubMed
-
- Levy L, Harris R. Effect of N-acetylcysteine on some aspects of cyclophosphamide induced toxicity and immuno-suppression. Biochem Pharmacol. 1977;26:1015–1020. - PubMed
-
- Cox Pj. Cyclophosphamide-cystitis identification of acrolein as the causative agent. Biochem Pharmacol. 1977;28:2045–2049. - PubMed
-
- Austin HA, Klippel JH, Balow JE, Le Riche NGH, Steinberg AD, Plotz PH, Decker JL. Therapy of lupus nephrits-controlled trial of prednisone and cytotoxic drugs. N Eng J Med. 1986;314:614–619. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

