Multifaceted approach to resveratrol bioactivity: Focus on antioxidant action, cell signaling and safety
- PMID: 20716933
- PMCID: PMC2952093
- DOI: 10.4161/oxim.3.2.11147
Multifaceted approach to resveratrol bioactivity: Focus on antioxidant action, cell signaling and safety
Abstract
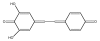
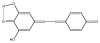
Resveratrol (RVT) is a naturally occurring trihydroxy stilbene that displays a wide spectrum of physiological activity. Its ability to behave therapeutically as a component of red wine has attracted wide attention. The phenol acts as a protective agent involving various body constituents. Most attention has been given to beneficial effects in insults involving cancer, aging, cardiovascular system, inflammation and the central nervous system. One of the principal modes of action appears to be as antioxidant. Other mechanistic pathways entail cell signaling, apoptosis and gene expression. There is an intriguing dichotomy in relation to pro-oxidant property. Also discussed are metabolism, receptor binding, rationale for safety and suggestions for future work. This is the first comprehensive review of RVT based on a broad, unifying mechanism.
Figures

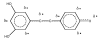


Similar articles
-
From French Paradox to cancer treatment: anti-cancer activities and mechanisms of resveratrol.Anticancer Agents Med Chem. 2014;14(6):806-25. doi: 10.2174/1871520614666140521121722. Anticancer Agents Med Chem. 2014. PMID: 24851878 Review.
-
Common pathways in health benefit properties of RSV in cardiovascular diseases, cancers and degenerative pathologies.Curr Pharm Biotechnol. 2015;16(3):219-44. doi: 10.2174/1389201016666150118132457. Curr Pharm Biotechnol. 2015. PMID: 25601605 Review.
-
Mechanism of cardioprotection by resveratrol, a phenolic antioxidant present in red wine (Review).Int J Mol Med. 2001 Jul;8(1):3-17. doi: 10.3892/ijmm.8.1.3. Int J Mol Med. 2001. PMID: 11408943 Review.
-
3,4,4'-Trihydroxy-trans-stilbene, an analogue of resveratrol, is a potent antioxidant and cytotoxic agent.Free Radic Res. 2011 Nov;45(11-12):1379-87. doi: 10.3109/10715762.2011.629199. Epub 2011 Oct 25. Free Radic Res. 2011. PMID: 21974738
-
Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans.Oxid Med Cell Longev. 2015;2015:837042. doi: 10.1155/2015/837042. Epub 2015 Jun 28. Oxid Med Cell Longev. 2015. PMID: 26221416 Free PMC article. Review.
Cited by
-
Resveratrol chemosensitizes HER-2-overexpressing breast cancer cells to docetaxel chemoresistance by inhibiting docetaxel-mediated activation of HER-2-Akt axis.Cell Death Discov. 2015 Dec 7;1:15061. doi: 10.1038/cddiscovery.2015.61. eCollection 2015. Cell Death Discov. 2015. PMID: 27551486 Free PMC article.
-
Resveratrol and diabetic cardiac function: focus on recent in vitro and in vivo studies.J Bioenerg Biomembr. 2012 Apr;44(2):281-96. doi: 10.1007/s10863-012-9429-0. J Bioenerg Biomembr. 2012. PMID: 22437738 Review.
-
Resveratrol Delivery from Implanted Cyclodextrin Polymers Provides Sustained Antioxidant Effect on Implanted Neural Probes.Int J Mol Sci. 2020 May 19;21(10):3579. doi: 10.3390/ijms21103579. Int J Mol Sci. 2020. PMID: 32438593 Free PMC article.
-
Antioxidants: powering the fight against fetal hypoxia.Philos Trans R Soc Lond B Biol Sci. 2025 Aug 21;380(1933):20240183. doi: 10.1098/rstb.2024.0183. Epub 2025 Aug 21. Philos Trans R Soc Lond B Biol Sci. 2025. PMID: 40836816 Free PMC article. Review.
-
Plant-derived anti-inflammatory compounds: hopes and disappointments regarding the translation of preclinical knowledge into clinical progress.Mediators Inflamm. 2014;2014:146832. doi: 10.1155/2014/146832. Epub 2014 May 29. Mediators Inflamm. 2014. PMID: 24987194 Free PMC article. Review.
References
-
- Udenigwe CC, Ramprasth VR, Aluko RE, Jones PJ. Potential of resveratrol in anticancer and anti-inflammatory therapy. Nutr Rev. 2008;66:445–454. - PubMed
-
- Kovacic P, Becvar LE. Mode of action of anti-infective agents: emphasis on oxidative stress and electron transfer. Curr Pharm Des. 2000;6:143–167. - PubMed
-
- Kovacic P, Osuna JA. Mechanisms of anticancer agents: emphasis on oxidative stress and electron transfer. Curr Pharm Des. 2000;6:277–309. - PubMed
-
- Kovacic P, Jacintho JD. Mechanism of carcinogenesis: focus on oxidative stress and electron transfer. Curr Med Chem. 2001;8:773–796. - PubMed
-
- Kovacic P, Jacintho JD. Reproductive toxins: pervasive theme of oxidative stress and electron transfer. Curr Med Chem. 2001;8:863–892. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

