Hyperactive sleeping beauty transposase enables persistent phenotypic correction in mice and a canine model for hemophilia B
- PMID: 20717103
- PMCID: PMC2990515
- DOI: 10.1038/mt.2010.169
Hyperactive sleeping beauty transposase enables persistent phenotypic correction in mice and a canine model for hemophilia B
Abstract
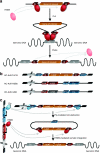
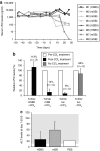
Sleeping Beauty (SB) transposase enables somatic integration of exogenous DNA in mammalian cells, but potency as a gene transfer vector especially in large mammals has been lacking. Herein, we show that hyperactive transposase system delivered by high-capacity adenoviral vectors (HC-AdVs) can result in somatic integration of a canine factor IX (cFIX) expression-cassette in canine liver, facilitating stabilized transgene expression and persistent haemostatic correction of canine hemophilia B with negligible toxicity. We observed stabilized cFIX expression levels during rapid cell cycling in mice and phenotypic correction of the bleeding diathesis in hemophilia B dogs for up to 960 days. In contrast, systemic administration of an inactive transposase system resulted in rapid loss of transgene expression and transient phenotypic correction. Notably, in dogs a higher viral dose of the active SB transposase system resulted into transient phenotypic correction accompanied by transient increase of liver enzymes. Molecular analysis of liver samples revealed SB-mediated integration and provide evidence that transgene expression was derived mainly from integrated vector forms. Demonstrating that a viral vector system can deliver clinically relevant levels of a therapeutic protein in a large animal model of human disease paves a new path toward the possible cure of genetic diseases.
Figures





Similar articles
-
Development of adenovirus hybrid vectors for Sleeping Beauty transposition in large mammals.Curr Gene Ther. 2011 Oct;11(5):363-74. doi: 10.2174/156652311797415890. Curr Gene Ther. 2011. PMID: 21888620 Review.
-
Integration profile and safety of an adenovirus hybrid-vector utilizing hyperactive sleeping beauty transposase for somatic integration.PLoS One. 2013 Oct 4;8(10):e75344. doi: 10.1371/journal.pone.0075344. eCollection 2013. PLoS One. 2013. PMID: 24124483 Free PMC article.
-
Helper-Independent Sleeping Beauty transposon-transposase vectors for efficient nonviral gene delivery and persistent gene expression in vivo.Mol Ther. 2003 Oct;8(4):654-65. doi: 10.1016/s1525-0016(03)00216-8. Mol Ther. 2003. PMID: 14529839
-
Prolonged Expression of Secreted Enzymes in Dogs After Liver-Directed Delivery of Sleeping Beauty Transposons: Implications for Non-Viral Gene Therapy of Systemic Disease.Hum Gene Ther. 2017 Jul;28(7):551-564. doi: 10.1089/hum.2017.004. Epub 2017 May 19. Hum Gene Ther. 2017. PMID: 28530135 Free PMC article.
-
Viral vector-mediated gene therapy for hemophilia B.Thromb Haemost. 1997 Jul;78(1):24-30. Thromb Haemost. 1997. PMID: 9198122 Review.
Cited by
-
Viral Vector-Based Delivery of CRISPR/Cas9 and Donor DNA for Homology-Directed Repair in an In Vitro Model for Canine Hemophilia B.Mol Ther Nucleic Acids. 2019 Mar 1;14:364-376. doi: 10.1016/j.omtn.2018.12.008. Epub 2018 Dec 20. Mol Ther Nucleic Acids. 2019. PMID: 30690229 Free PMC article.
-
Constitutive and Inducible Systems for Genetic In Vivo Modification of Mouse Hepatocytes Using Hydrodynamic Tail Vein Injection.J Vis Exp. 2018 Feb 2;(132):56613. doi: 10.3791/56613. J Vis Exp. 2018. PMID: 29443066 Free PMC article.
-
The Evolution of Gene Therapy in the Treatment of Metabolic Liver Diseases.Genes (Basel). 2020 Aug 10;11(8):915. doi: 10.3390/genes11080915. Genes (Basel). 2020. PMID: 32785089 Free PMC article. Review.
-
The use of adenoviral vectors in gene therapy and vaccine approaches.Genet Mol Biol. 2022 Oct 7;45(3 Suppl 1):e20220079. doi: 10.1590/1678-4685-GMB-2022-0079. eCollection 2022. Genet Mol Biol. 2022. PMID: 36206378 Free PMC article.
-
Preclinical and clinical advances in transposon-based gene therapy.Biosci Rep. 2017 Dec 5;37(6):BSR20160614. doi: 10.1042/BSR20160614. Print 2017 Dec 22. Biosci Rep. 2017. PMID: 29089466 Free PMC article. Review.
References
-
- Kay MA, Rothenberg S, Landen CN, Bellinger DA, Leland F, Toman C, et al. In vivo gene therapy of hemophilia B: sustained partial correction in factor IX-deficient dogs. Science. 1993;262:117–119. - PubMed
-
- Brown BD, Cantore A, Annoni A, Sergi LS, Lombardo A, Della Valle P, et al. A microRNA-regulated lentiviral vector mediates stable correction of hemophilia B mice. Blood. 2007;110:4144–4152. - PubMed
-
- Vandendriessche T, Thorrez L, Acosta-Sanchez A, Petrus I, Wang L, Ma L, et al. Efficacy and safety of adeno-associated viral vectors based on serotype 8 and 9 vs. lentiviral vectors for hemophilia B gene therapy. J Thromb Haemost. 2007;5:16–24. - PubMed
-
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

