An intimate liaison: spatial organization of the endoplasmic reticulum-mitochondria relationship
- PMID: 20717141
- PMCID: PMC2924651
- DOI: 10.1038/emboj.2010.177
An intimate liaison: spatial organization of the endoplasmic reticulum-mitochondria relationship
Abstract
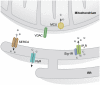
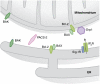
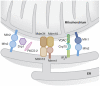
Organelle localization is often crucial to properly modulate cellular functions and signalling cascades. For example, the distribution of organelles in axons is crucial for their function and is dysregulated in several diseases. Similarly, relative positioning of two or more organelles is also important to perform certain specialized processes. Perhaps, the best-known form of interorganellar organization is that between endoplasmic reticulum (ER) and mitochondria. Close communication between these two compartments has been observed for a long time. Recent evidence suggests that this is the basis for a bidirectional communication regulating a number of physiological processes ranging from mitochondrial energy and lipid metabolism to Ca(2+) signalling and cell death. The recent discovery of some of the molecular mediators of the tethering already allowed to extend the function of this paradigmatic spatial organization to previously unexpected functions, and will foster future research to explore it in cellular signalling cascades as well as in disease.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Amarilio R, Ramachandran S, Sabanay H, Lev S (2005) Differential regulation of endoplasmic reticulum structure through VAP-Nir protein interaction. J Biol Chem 280: 5934–5944 - PubMed
-
- Annis MG, Zamzami N, Zhu W, Penn LZ, Kroemer G, Leber B, Andrews DW (2001) Endoplasmic reticulum localized Bcl-2 prevents apoptosis when redistribution of cytochrome c is a late event. Oncogene 20: 1939–1952 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

