Cellular responses to extracellular guidance cues
- PMID: 20717143
- PMCID: PMC2924648
- DOI: 10.1038/emboj.2010.170
Cellular responses to extracellular guidance cues
Abstract
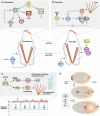
Extracellular guidance cues have a key role in orchestrating cell behaviour. They can take many forms, including soluble and cell-bound ligands (proteins, lipids, peptides or small molecules) and insoluble matrix substrates, but to act as guidance cues, they must be presented to the cell in a spatially restricted manner. Cells that recognize such cues respond by activating intracellular signal transduction pathways in a spatially restricted manner and convert the extracellular information into intracellular polarity. Although extracellular cues influence a broad range of cell polarity decisions, such as mitotic spindle orientation during asymmetric cell division, or the establishment of apical-basal polarity in epithelia, this review will focus specifically on guidance cues that promote cell migration (chemotaxis), or localized cell shape changes (chemotropism).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
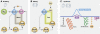

Similar articles
-
Myosin 2-Induced Mitotic Rounding Enables Columnar Epithelial Cells to Interpret Cortical Spindle Positioning Cues.Curr Biol. 2017 Nov 6;27(21):3350-3358.e3. doi: 10.1016/j.cub.2017.09.039. Epub 2017 Oct 26. Curr Biol. 2017. PMID: 29107549
-
Establishment and maintenance of cell polarity during leukocyte chemotaxis.Cell Adh Migr. 2007 Apr-Jun;1(2):69-76. doi: 10.4161/cam.1.2.4547. Epub 2007 Apr 6. Cell Adh Migr. 2007. PMID: 19329880 Free PMC article. Review.
-
Mechanical guidance of cell migration: lessons from chemotaxis.Curr Opin Cell Biol. 2013 Oct;25(5):543-9. doi: 10.1016/j.ceb.2013.04.010. Epub 2013 May 28. Curr Opin Cell Biol. 2013. PMID: 23726023 Review.
-
Extracellular Regulation of the Mitotic Spindle and Fate Determinants Driving Asymmetric Cell Division.Results Probl Cell Differ. 2017;61:351-373. doi: 10.1007/978-3-319-53150-2_16. Results Probl Cell Differ. 2017. PMID: 28409313 Free PMC article. Review.
-
The principles of directed cell migration.Nat Rev Mol Cell Biol. 2021 Aug;22(8):529-547. doi: 10.1038/s41580-021-00366-6. Epub 2021 May 14. Nat Rev Mol Cell Biol. 2021. PMID: 33990789 Free PMC article. Review.
Cited by
-
Phosphorylated Rho-GDP directly activates mTORC2 kinase towards AKT through dimerization with Ras-GTP to regulate cell migration.Nat Cell Biol. 2019 Jul;21(7):867-878. doi: 10.1038/s41556-019-0348-8. Epub 2019 Jul 1. Nat Cell Biol. 2019. PMID: 31263268 Free PMC article.
-
SMRT analysis of MTOC and nuclear positioning reveals the role of EB1 and LIC1 in single-cell polarization.J Cell Sci. 2011 Dec 15;124(Pt 24):4267-85. doi: 10.1242/jcs.091231. Epub 2011 Dec 22. J Cell Sci. 2011. PMID: 22193958 Free PMC article.
-
Cell cycle regulation of Rho signaling pathways.Cell Cycle. 2012 Aug 15;11(16):3003-10. doi: 10.4161/cc.21088. Epub 2012 Jul 24. Cell Cycle. 2012. PMID: 22825247 Free PMC article. Review.
-
Cdc42 and the guanine nucleotide exchange factors Ect2 and trio mediate Fn14-induced migration and invasion of glioblastoma cells.Mol Cancer Res. 2012 Jul;10(7):958-68. doi: 10.1158/1541-7786.MCR-11-0616. Epub 2012 May 9. Mol Cancer Res. 2012. PMID: 22571869 Free PMC article.
-
Wnt5a signaling promotes apical and basolateral polarization of single epithelial cells.Mol Biol Cell. 2013 Dec;24(23):3764-74. doi: 10.1091/mbc.E13-07-0357. Epub 2013 Oct 2. Mol Biol Cell. 2013. PMID: 24088568 Free PMC article.
References
-
- Aman A, Piotrowski T (2010) Cell migration during morphogenesis. Dev Biol 341: 20–33 - PubMed
-
- Andrew N, Insall RH (2007) Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat Cell Biol 9: 193–200 - PubMed
-
- Arimura N, Inagaki N, Chihara K, Menager C, Nakamura N, Amano M, Iwamatsu A, Goshima Y, Kaibuchi K (2000) Phosphorylation of collapsin response mediator protein-2 by Rho-kinase. Evidence for two separate signaling pathways for growth cone collapse. J Biol Chem 275: 23973–23980 - PubMed
-
- Barberis D, Artigiani S, Casazza A, Corso S, Giordano S, Love CA, Jones EY, Comoglio PM, Tamagnone L (2004) Plexin signaling hampers integrin-based adhesion, leading to Rho-kinase independent cell rounding, and inhibiting lamellipodia extension and cell motility. FASEB J 18: 592–594 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

