A Diels-Alder Route to Angularly Functionalized Bicyclic Structures
- PMID: 20717474
- PMCID: PMC2921539
- DOI: 10.1016/j.tet.2010.04.094
A Diels-Alder Route to Angularly Functionalized Bicyclic Structures
Abstract
A Diels-Alder based route to trans-fused angularly functionalized bicyclic structures has been developed. This transformation features the use of a tetrasubstituted dienophile in the cycloaddition step.
Figures

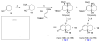

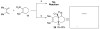
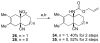
Similar articles
-
Diels-Alder routes to angularly halogenated cis-fused bicyclic ketones: Readily accessible cyclynone intermediates.Tetrahedron Lett. 2010 Sep 1;51(35):4653-4654. doi: 10.1016/j.tetlet.2010.06.135. Tetrahedron Lett. 2010. PMID: 20835369 Free PMC article.
-
A Direct Organocatalytic Enantioselective Route to Functionalized trans-Diels-Alder Products Having the Norcarane Scaffold.Angew Chem Int Ed Engl. 2021 Aug 9;60(33):18318-18327. doi: 10.1002/anie.202106598. Epub 2021 Jul 9. Angew Chem Int Ed Engl. 2021. PMID: 34080269
-
Base-catalyzed tandem Michael/dehydro-Diels-Alder reaction of α,α-dicyanoolefins with electron-deficient 1,3-conjugated enynes: a facile entry to angularly fused polycycles.Chemistry. 2014 Jan 7;20(2):399-404. doi: 10.1002/chem.201302960. Epub 2013 Dec 2. Chemistry. 2014. PMID: 24302563
-
Progress in Lewis-Acid-Templated Diels-Alder Reactions.Molecules. 2024 Mar 6;29(5):1187. doi: 10.3390/molecules29051187. Molecules. 2024. PMID: 38474699 Free PMC article. Review.
-
Nitroso Diels-Alder Cycloadducts Derived From N-Acyl-1,2-dihydropyridines as a New Platform to Molecular Diversity.Molecules. 2020 Jan 28;25(3):563. doi: 10.3390/molecules25030563. Molecules. 2020. PMID: 32012951 Free PMC article. Review.
Cited by
-
Synthesis of angularly substituted trans-fused hydroindanes by convergent coupling of acyclic precursors.J Am Chem Soc. 2014 Jun 11;136(23):8209-12. doi: 10.1021/ja504374j. Epub 2014 May 29. J Am Chem Soc. 2014. PMID: 24856045 Free PMC article.
-
Further Expansion of the trans-Diels-Alder Paradigm: Reductive Alkylation of α-Cyanoketones.Tetrahedron Lett. 2011 Aug 3;52(31):3957-3959. doi: 10.1016/j.tetlet.2011.05.110. Tetrahedron Lett. 2011. PMID: 21808427 Free PMC article.
-
Pattern recognition analysis in complex molecule synthesis and the preparation of iso-Diels-Alder motifs.Proc Natl Acad Sci U S A. 2013 Jul 2;110(27):10904-9. doi: 10.1073/pnas.1309795110. Epub 2013 Jun 19. Proc Natl Acad Sci U S A. 2013. PMID: 23784777 Free PMC article.
-
A straightforward route to functionalized trans-Diels-Alder motifs.J Am Chem Soc. 2010 Oct 20;132(41):14330-3. doi: 10.1021/ja1073855. J Am Chem Soc. 2010. PMID: 20863105 Free PMC article.
References
-
-
For recent reviews, see: Trost BM, Jiang C. Synthesis. 2006:369–396. Douglas C, Overman LE. Proc. Natl. Acad. Sci. U.S.A. 2004;101:5363–5367. Corey EJ, Guzman-Perez A. Angew. Chem. Int. Ed. 1998;37:388–401.
-
-
-
For examples of Diels-Alder reactions of cyclohexenecarboxaldehyde or its corresponding acetal to obtain cis-fused bicyclic ring system with all-carbon quaternary center on the bridgehead, see: Szmuszkowicz J, Bergmann ED. Bull. Res. Counc. Israel. 1953;3:93–95. Bergmann ED, Becker A. J. Am. Chem. Soc. 1959;81:221–225. Lee JH, Kim WH, Danishefsky SJ. Tetrahedron Lett. 2010;51:1252–1253.
-
-
-
Most examples of tetrasubstituted cyclic dienophiles are limited to quinone and maleic anhydride derivatives. For examples, see: Kwon O, Park SB, Schreiber SL. J. Am. Chem. Soc. 2002;124:13402–13404. Nicolaou KC, Vassilikogiannakis G, Mägerlein W, Kranich R. Angew. Chem. Int. Ed. 2001;40:2482–2486.
-
-
- Fuji K, Tanaka K, Abe H, Matsumoto K, Taga T, Miwa Y. Tetrahedron: Asymmetry. 1992;3:609–612.
- Piaz VD, Giovannoni MP, Ciciani G, Giomi D, Nesi R. Tetrahedron Lett. 1993;34:161–162.
- Nesi R, Giomi D, Papaleo S, Corti M. J. Org. Chem. 1990;55:1227–1230.
Grants and funding
LinkOut - more resources
Full Text Sources
