Sequential events in the irreversible thermal denaturation of human brain-type creatine kinase by spectroscopic methods
- PMID: 20717523
- PMCID: PMC2920553
- DOI: 10.3390/ijms11072584
Sequential events in the irreversible thermal denaturation of human brain-type creatine kinase by spectroscopic methods
Abstract
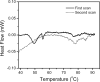
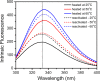
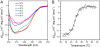
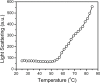
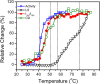
The non-cooperative or sequential events which occur during protein thermal denaturation are closely correlated with protein folding, stability, and physiological functions. In this research, the sequential events of human brain-type creatine kinase (hBBCK) thermal denaturation were studied by differential scanning calorimetry (DSC), CD, and intrinsic fluorescence spectroscopy. DSC experiments revealed that the thermal denaturation of hBBCK was calorimetrically irreversible. The existence of several endothermic peaks suggested that the denaturation involved stepwise conformational changes, which were further verified by the discrepancy in the transition curves obtained from various spectroscopic probes. During heating, the disruption of the active site structure occurred prior to the secondary and tertiary structural changes. The thermal unfolding and aggregation of hBBCK was found to occur through sequential events. This is quite different from that of muscle-type CK (MMCK). The results herein suggest that BBCK and MMCK undergo quite dissimilar thermal unfolding pathways, although they are highly conserved in the primary and tertiary structures. A minor difference in structure might endow the isoenzymes dissimilar local stabilities in structure, which further contribute to isoenzyme-specific thermal stabilities.
Keywords: differential scanning calorimetry; human brain-type creatine kinase; intrinsic fluorescence; stepwise transitions; thermal denaturation.
Figures


Similar articles
-
Trehalose has a protective effect on human brain-type creatine kinase during thermal denaturation.Appl Biochem Biotechnol. 2011 Sep;165(2):476-84. doi: 10.1007/s12010-011-9266-3. Epub 2011 Apr 26. Appl Biochem Biotechnol. 2011. PMID: 21519905
-
Isoenzyme-specific thermostability of human cytosolic creatine kinase.Int J Biol Macromol. 2010 Jul 1;47(1):27-32. doi: 10.1016/j.ijbiomac.2010.03.025. Epub 2010 Apr 8. Int J Biol Macromol. 2010. PMID: 20381520
-
Studies on the stability of creatine kinase isozymes.Biochem Cell Biol. 2003 Feb;81(1):9-16. doi: 10.1139/o02-171. Biochem Cell Biol. 2003. PMID: 12683631
-
Irreversible denaturation of maltodextrin glucosidase studied by differential scanning calorimetry, circular dichroism, and turbidity measurements.PLoS One. 2014 Dec 30;9(12):e115877. doi: 10.1371/journal.pone.0115877. eCollection 2014. PLoS One. 2014. PMID: 25548918 Free PMC article.
-
Dissociative mechanism for irreversible thermal denaturation of oligomeric proteins.Biophys Rev. 2016 Dec;8(4):397-407. doi: 10.1007/s12551-016-0220-z. Epub 2016 Oct 17. Biophys Rev. 2016. PMID: 28510015 Free PMC article. Review.
Cited by
-
Dissimilarity in the folding of human cytosolic creatine kinase isoenzymes.PLoS One. 2011;6(9):e24681. doi: 10.1371/journal.pone.0024681. Epub 2011 Sep 9. PLoS One. 2011. PMID: 21931810 Free PMC article.
-
Improved scFv Anti-LOX-1 Binding Activity by Fusion with LOX-1-Binding Peptides.Biomed Res Int. 2017;2017:8946935. doi: 10.1155/2017/8946935. Epub 2017 Sep 28. Biomed Res Int. 2017. PMID: 29094051 Free PMC article.
-
Effect of Protein Structure on Evolution of Cotranslational Folding.Biophys J. 2020 Sep 15;119(6):1123-1134. doi: 10.1016/j.bpj.2020.06.037. Epub 2020 Aug 12. Biophys J. 2020. PMID: 32857962 Free PMC article.
-
A single residue substitution accounts for the significant difference in thermostability between two isoforms of human cytosolic creatine kinase.Sci Rep. 2016 Feb 16;6:21191. doi: 10.1038/srep21191. Sci Rep. 2016. PMID: 26879258 Free PMC article.
-
Modeling protein folding in vivo.Biol Direct. 2018 Jul 6;13(1):13. doi: 10.1186/s13062-018-0217-6. Biol Direct. 2018. PMID: 29980221 Free PMC article.
References
-
- Jaenicke R. Stability and folding of domain proteins. Prog. Biophys. Mol. Biol. 1999;71:155–241. - PubMed
-
- Jaenicke R, Lilie H, Matthews CR. Folding and association of oligomeric and multimeric proteins. Adv. Protein Chem. 2000;53:329–362. - PubMed
-
- Burgess AW, Scheraga HA. A hypothesis for the pathway of the thermally-induced unfolding of bovine pancreatic ribonuclease. J. Theor. Biol. 1975;53:403–420. - PubMed
-
- Matheson RR, Jr, Scheraga HA. Steps in the pathway of the thermal unfolding of ribonuclease A. A nonspecific photochemical surface-labeling study. Biochemistry. 1979;18:2437–2445. - PubMed
-
- Matheson RR, Jr, Scheraga HA. Steady-state kinetic study of action of ribonuclease A, involving a conformational change between 30 and 40 degrees C. Biochemistry. 1979;18:2446–2450. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

