Chelation in metal intoxication
- PMID: 20717537
- PMCID: PMC2922724
- DOI: 10.3390/ijerph7072745
Chelation in metal intoxication
Abstract
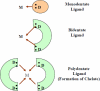
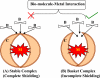
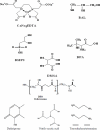
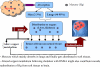
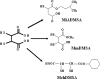
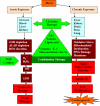
Chelation therapy is the preferred medical treatment for reducing the toxic effects of metals. Chelating agents are capable of binding to toxic metal ions to form complex structures which are easily excreted from the body removing them from intracellular or extracellular spaces. 2,3-Dimercaprol has long been the mainstay of chelation therapy for lead or arsenic poisoning, however its serious side effects have led researchers to develop less toxic analogues. Hydrophilic chelators like meso-2,3-dimercaptosuccinic acid effectively promote renal metal excretion, but their ability to access intracellular metals is weak. Newer strategies to address these drawbacks like combination therapy (use of structurally different chelating agents) or co-administration of antioxidants have been reported recently. In this review we provide an update of the existing chelating agents and the various strategies available for the treatment of heavy metals and metalloid intoxications.
Keywords: antioxidant; chelating agents; combination therapy; heavy metals; monoesters; oxidative stress; succimer.
Figures





Similar articles
-
Chemistry, Pharmacology, and Toxicology of Monoisoamyl Dimercaptosuccinic Acid: A Chelating Agent for Chronic Metal Poisoning.Chem Res Toxicol. 2022 Oct 17;35(10):1701-1719. doi: 10.1021/acs.chemrestox.2c00129. Epub 2022 Aug 16. Chem Res Toxicol. 2022. PMID: 35972774 Review.
-
Chemical and biological considerations in the treatment of metal intoxications by chelating agents.Mini Rev Med Chem. 2004 Jan;4(1):11-21. doi: 10.2174/1389557043487583. Mini Rev Med Chem. 2004. PMID: 14754439 Review.
-
Arsenic induced oxidative stress and the role of antioxidant supplementation during chelation: a review.J Environ Biol. 2007 Apr;28(2 Suppl):333-47. J Environ Biol. 2007. PMID: 17929749 Review.
-
Arsenic and lead induced free radical generation and their reversibility following chelation.Cell Mol Biol (Noisy-le-grand). 2007 Apr 15;53(1):26-47. Cell Mol Biol (Noisy-le-grand). 2007. PMID: 17519110 Review.
-
Strategies for safe and effective therapeutic measures for chronic arsenic and lead poisoning.J Occup Health. 2005 Jan;47(1):1-21. doi: 10.1539/joh.47.1. J Occup Health. 2005. PMID: 15703449 Review.
Cited by
-
Therapeutic Potential of Iron Chelators in Viral Diseases: A Systematic Review.Curr Med Chem. 2024;31(27):4383-4391. doi: 10.2174/0109298673259596231211113211. Curr Med Chem. 2024. PMID: 38321902
-
Zinc Deficiency in Men Over 50 and Its Implications in Prostate Disorders.Front Oncol. 2020 Aug 6;10:1293. doi: 10.3389/fonc.2020.01293. eCollection 2020. Front Oncol. 2020. PMID: 32850402 Free PMC article. Review.
-
Comparative analysis on the effect of palm oil (Elaeis guineensis) in reducing cadmium and lead accumulation in liver of Wistar rats.Pharmacognosy Res. 2012 Oct;4(4):214-8. doi: 10.4103/0974-8490.102266. Pharmacognosy Res. 2012. PMID: 23225965 Free PMC article.
-
Nano Selenium-Enriched Probiotics as Functional Food Products against Cadmium Liver Toxicity.Materials (Basel). 2021 Apr 27;14(9):2257. doi: 10.3390/ma14092257. Materials (Basel). 2021. PMID: 33925590 Free PMC article.
-
Upregulation of the host SLC11A1 gene by Clostridium difficile toxin B facilitates glucosylation of Rho GTPases and enhances toxin lethality.Infect Immun. 2013 Aug;81(8):2724-32. doi: 10.1128/IAI.01177-12. Epub 2013 May 20. Infect Immun. 2013. PMID: 23690404 Free PMC article.
References
-
- Morgan T, Gilbert T, Drew, Harry DK. CLXII—Researches on residual affinity and co-ordination. Part II Acetyl acetones of selenium and tellurium. J. Chem. Soc. 1920;117:1456–1465.
-
- Andersen O. Principles and recent developments in chelation treatment of metal intoxication. Chem. Rev. 1999;99:2683–2710. - PubMed
-
- Jones MM. Design of new chelating agents for removal of intracellular toxic metals. In: Kauffman GB, editor. Coordination Chemistry: A Century of Progress. The American Chemical Society; Washington, DC, USA: 1994. pp. 427–438.
-
- Baum CR. Treatment of mercury intoxication. Curr. Opin. Pediatr. 1999;11:265–268. - PubMed
-
- Guldager B, Jorgensen PJ, Grandjean P. Metal excretion and magnesium retention in patients with intermittent claudication treated with intravenous disodium EDTA. Clin. Chem. 1996;42:1938–1942. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

