Chelation in metal intoxication
- PMID: 20717537
- PMCID: PMC2922724
- DOI: 10.3390/ijerph7072745
Chelation in metal intoxication
Abstract
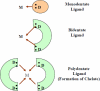
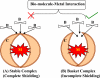
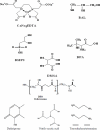
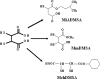
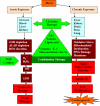
Chelation therapy is the preferred medical treatment for reducing the toxic effects of metals. Chelating agents are capable of binding to toxic metal ions to form complex structures which are easily excreted from the body removing them from intracellular or extracellular spaces. 2,3-Dimercaprol has long been the mainstay of chelation therapy for lead or arsenic poisoning, however its serious side effects have led researchers to develop less toxic analogues. Hydrophilic chelators like meso-2,3-dimercaptosuccinic acid effectively promote renal metal excretion, but their ability to access intracellular metals is weak. Newer strategies to address these drawbacks like combination therapy (use of structurally different chelating agents) or co-administration of antioxidants have been reported recently. In this review we provide an update of the existing chelating agents and the various strategies available for the treatment of heavy metals and metalloid intoxications.
Keywords: antioxidant; chelating agents; combination therapy; heavy metals; monoesters; oxidative stress; succimer.
Figures





References
-
- Morgan T, Gilbert T, Drew, Harry DK. CLXII—Researches on residual affinity and co-ordination. Part II Acetyl acetones of selenium and tellurium. J. Chem. Soc. 1920;117:1456–1465.
-
- Andersen O. Principles and recent developments in chelation treatment of metal intoxication. Chem. Rev. 1999;99:2683–2710. - PubMed
-
- Jones MM. Design of new chelating agents for removal of intracellular toxic metals. In: Kauffman GB, editor. Coordination Chemistry: A Century of Progress. The American Chemical Society; Washington, DC, USA: 1994. pp. 427–438.
-
- Baum CR. Treatment of mercury intoxication. Curr. Opin. Pediatr. 1999;11:265–268. - PubMed
-
- Guldager B, Jorgensen PJ, Grandjean P. Metal excretion and magnesium retention in patients with intermittent claudication treated with intravenous disodium EDTA. Clin. Chem. 1996;42:1938–1942. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

