Endogenous patterns of mechanical stress are required for branching morphogenesis
- PMID: 20717570
- PMCID: PMC3074564
- DOI: 10.1039/c0ib00040j
Endogenous patterns of mechanical stress are required for branching morphogenesis
Abstract
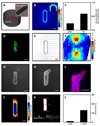
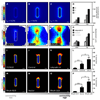
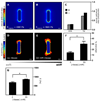
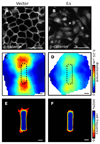
Spatial patterning of cell behaviors establishes the regional differences within tissues that collectively develop branched organs into their characteristic treelike shapes. Here we show that the pattern of branching morphogenesis of three-dimensional (3D) engineered epithelial tissues is controlled in part by gradients of endogenous mechanical stress. We used microfabrication to build model mammary epithelial tissues of defined geometry that branched in a stereotyped pattern when induced with growth factors. Branches initiated from sites of high mechanical stress within the tissues, as predicted numerically and measured directly using 3D traction force microscopy. Branch sites were defined by activation of focal adhesion kinase (FAK), inhibition of which disrupted morphogenesis. Stress, FAK activation, and branching were all altered by manipulating cellular contractility, matrix stiffness, intercellular cohesion and tissue geometry. These data suggest that the pattern and magnitude of mechanical stress across epithelial tissues cooperate with biochemical signals to specify branching pattern.
Figures
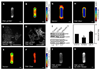
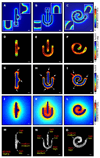
References
-
- Affolter M, Bellusci S, Itoh N, Shilo B, Thiery JP, Werb Z. Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev. Cell. 2003;4:11–18. - PubMed
-
- Davies JA. Branching morphogenesis. New York: Landes Bioscience; 2006.
-
- Affolter M, Zeller R, Caussinus E. Tissue remodelling through branching morphogenesis. Nat. Rev. Mol. Cell Biol. 2009;10:831–842. - PubMed
-
- Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006;7:211–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

