Survivin is released from cancer cells via exosomes
- PMID: 20717727
- PMCID: PMC3174681
- DOI: 10.1007/s10495-010-0534-4
Survivin is released from cancer cells via exosomes
Abstract
Inhibitor of apoptosis (IAP) and Heat shock proteins (HSPs) provide assistance in protecting cells from stresses of hypoxia, imbalanced pH, and altered metabolic and redox states commonly found in the microenvironmental mixture of tumor and nontumor cells. HSPs are upregulated, cell-surface displayed and released extracellularly in some types of tumors, a finding that until now was not shared by members of the IAP family. The IAP Survivin has been implicated in apoptosis inhibition and the regulation of mitosis in cancer cells. Survivin exists in a number of subcellular locations such as the mitochondria, cytoplasm, nucleus, and most recently, the extracellular space. Our previous work showing that extracellular survivin was able to enhance cellular proliferation, survival and tumor cell invasion provides evidence that Survivin might be secreted via an unidentified exocytotic pathway. In the present study, we describe for the first time the exosome-release of Survivin to the extracellular space both basally and after proton irradiation-induced stress. To examine whether exosomes contributed to Survivin release from cancer cells, exosomes were purified from HeLa cervical carcinoma cells and exosome quantity and Survivin content were determined. We demonstrate that although proton irradiation does not influence the exosomal secretory rate, the Survivin content of exosomes isolated from HeLa cells treated with a sublethal dose of proton irradiation (3 Gy) is significantly higher than control. These data identify a novel secretory pathway by which Survivin can be actively released from cells in both the basal and stress-induced state.
Conflict of interest statement
Figures

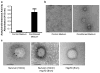

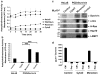

References
-
- Ohtani H. Stromal reaction in cancer tissue: pathophysiologic significance of the expression of matrix-degrading enzymes in relation to matrix turnover and immune/inflammatory reactions. Pathol Int. 1998;48:1–9. - PubMed
-
- Eustace BK, Jay DG. Extracellular roles for the molecular chaperone, hsp90. Cell Cycle. 2004;3:1098–1100. - PubMed
-
- Radons J, Multhoff G. Immunostimulatory functions of membrane-bound and exported heat shock protein 70. Exerc Immunol Rev. 2005;11:17–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

