Targeting O⁶-methylguanine-DNA methyltransferase with specific inhibitors as a strategy in cancer therapy
- PMID: 20717836
- PMCID: PMC11115711
- DOI: 10.1007/s00018-010-0491-7
Targeting O⁶-methylguanine-DNA methyltransferase with specific inhibitors as a strategy in cancer therapy
Abstract
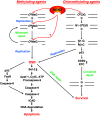
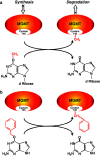
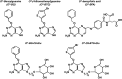
O (6)-methylguanine-DNA methyltransferase (MGMT) repairs the cancer chemotherapy-relevant DNA adducts, O (6)-methylguanine and O (6)-chloroethylguanine, induced by methylating and chloroethylating anticancer drugs, respectively. These adducts are cytotoxic, and given the overwhelming evidence that MGMT is a key factor in resistance, strategies for inactivating MGMT have been pursued. A number of drugs have been shown to inactivate MGMT in cells, human tumour models and cancer patients, and O (6)-benzylguanine and O (6)-[4-bromothenyl]guanine have been used in clinical trials. While these agents show no side effects per se, they also inactivate MGMT in normal tissues and hence exacerbate the toxic side effects of the alkylating drugs, requiring dose reduction. This might explain why, in any of the reported trials, the outcome has not been improved by their inclusion. It is, however, anticipated that, with the availability of tumour targeting strategies and hematopoetic stem cell protection, MGMT inactivators hold promise for enhancing the effectiveness of alkylating agent chemotherapy.
Figures
Similar articles
-
The specific role of O6-methylguanine-DNA methyltransferase inhibitors in cancer chemotherapy.Future Med Chem. 2018 Aug 1;10(16):1971-1996. doi: 10.4155/fmc-2018-0069. Epub 2018 Jul 13. Future Med Chem. 2018. PMID: 30001630 Review.
-
S-alkylthiolation of O6-methylguanine-DNA-methyltransferase (MGMT) to sensitize cancer cells to anticancer therapy.Expert Opin Ther Targets. 2007 Mar;11(3):349-61. doi: 10.1517/14728222.11.3.349. Expert Opin Ther Targets. 2007. PMID: 17298293 Review.
-
Targeted modulation of MGMT: clinical implications.Clin Cancer Res. 2006 Jan 15;12(2):328-31. doi: 10.1158/1078-0432.CCR-05-2543. Clin Cancer Res. 2006. PMID: 16428468 Review.
-
Posttranslational Regulation of O(6)-Methylguanine-DNA Methyltransferase (MGMT) and New Opportunities for Treatment of Brain Cancers.Mini Rev Med Chem. 2016;16(6):455-64. doi: 10.2174/1389557515666150722101046. Mini Rev Med Chem. 2016. PMID: 26202203 Review.
-
O(6)-Methylguanine-DNA methyltransferase (MGMT) in normal tissues and tumors: enzyme activity, promoter methylation and immunohistochemistry.Biochim Biophys Acta. 2011 Dec;1816(2):179-90. doi: 10.1016/j.bbcan.2011.06.002. Epub 2011 Jul 1. Biochim Biophys Acta. 2011. PMID: 21745538 Review.
Cited by
-
Completeness of required site-specific factors for brain and CNS tumors in the Surveillance, Epidemiology and End Results (SEER) 18 database (2004-2012, varying).J Neurooncol. 2016 Oct;130(1):31-42. doi: 10.1007/s11060-016-2217-7. Epub 2016 Jul 14. J Neurooncol. 2016. PMID: 27418206 Free PMC article.
-
Progression of O⁶-methylguanine-DNA methyltransferase and temozolomide resistance in cancer research.Mol Biol Rep. 2014 Oct;41(10):6659-65. doi: 10.1007/s11033-014-3549-z. Epub 2014 Jul 3. Mol Biol Rep. 2014. PMID: 24990698 Review.
-
Influence of DNA repair on nonlinear dose-responses for mutation.Toxicol Sci. 2013 Mar;132(1):87-95. doi: 10.1093/toxsci/kfs341. Epub 2013 Jan 3. Toxicol Sci. 2013. PMID: 23288051 Free PMC article.
-
DNA methylation and cancer diagnosis.Int J Mol Sci. 2013 Jul 18;14(7):15029-58. doi: 10.3390/ijms140715029. Int J Mol Sci. 2013. PMID: 23873296 Free PMC article. Review.
-
Insight into the cooperative DNA binding of the O⁶-alkylguanine DNA alkyltransferase.DNA Repair (Amst). 2014 Aug;20:14-22. doi: 10.1016/j.dnarep.2014.01.006. Epub 2014 Feb 16. DNA Repair (Amst). 2014. PMID: 24553127 Free PMC article. Review.
References
-
- Kleihues P, Magee PN. Alkylation of rat brain nucleic acids by N-methyl-N-nitrosourea and methyl methanesulphonate. J Neurochem. 1973;20:595–606. - PubMed
-
- Skipper HE, Schabel FM, Jr, Trader MW, Thomson JR. Experimental evaluation of potential anticancer agents. VI. Anatomical distribution of leukemic cells and failure of chemotherapy. Cancer Res. 1961;21:1154–1164. - PubMed
-
- Ludlum DB. DNA alkylation by the haloethylnitrosoureas: nature of modifications produced and their enzymatic repair or removal. Mutat Res. 1990;233:117–126. - PubMed
-
- Goldstein M, Roos WP, Kaina B. Apoptotic death induced by the cyclophosphamide analogue mafosfamide in human lymphoblastoid cells: contribution of DNA replication, transcription inhibition and Chk/p53 signaling. Toxicol Appl Pharmacol. 2008;229:20–32. - PubMed
-
- Preuss I, Thust R, Kaina B. Protective effect of O6-methylguanine-DNA methyltransferase (MGMT) on the cytotoxic and recombinogenic activity of different antineoplastic drugs. Int J Cancer. 1996;65:506–512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

