Pharmacogenetics of the mycophenolic acid targets inosine monophosphate dehydrogenases IMPDH1 and IMPDH2: gene sequence variation and functional genomics
- PMID: 20718729
- PMCID: PMC3010569
- DOI: 10.1111/j.1476-5381.2010.00987.x
Pharmacogenetics of the mycophenolic acid targets inosine monophosphate dehydrogenases IMPDH1 and IMPDH2: gene sequence variation and functional genomics
Abstract
Background and purpose: Inosine monophosphate dehydrogenases, encoded by IMPDH1 and IMPDH2, are targets for the important immunosuppressive drug, mycophenolic acid (MPA). Variation in MPA response may result, in part, from genetic variation in IMPDH1 and IMPDH2.
Experimental approach: We resequenced IMPDH1 and IMPDH2 using DNA from 288 individuals from three ethnic groups and performed functional genomic studies of the sequence variants observed.
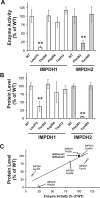
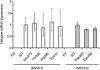
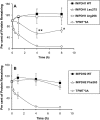
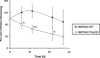
Key results: We identified 73 single nucleotide polymorphisms (SNPs) in IMPDH1, 59 novel, and 25 SNPs, 24 novel, in IMPDH2. One novel IMPDH1 allozyme (Leu275) had 10.2% of the wild-type activity as a result of accelerated protein degradation. Decreased activity of the previously reported IMPDH2 Phe263 allozyme was primarily due to decreased protein quantity, also with accelerated degradation. These observations with regard to the functional implications of variant allozymes were supported by the IMPDH1 and IMPDH2 X-ray crystal structures. A novel IMPDH2 intron 1 SNP, G > C IVS1(93), was associated with decreased mRNA quantity, possibly because of altered transcription.
Conclusions and implications: These results provide insight into the nature and extent of sequence variation in the IMPDH1 and IMPDH2 genes. They also describe the influence of gene sequence variation that alters the encoded amino acids on IMPDH function and provide a foundation for future translational studies designed to correlate sequence variation in these genes with outcomes in patients treated with MPA.
Figures


References
-
- Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47:85–118. - PubMed
-
- Carr SF, Papp E, Wu JC, Natsumeda Y. Characterization of human type I and type II IMP dehydrogenases. J Biol Chem. 1993;268:27286–27290. - PubMed
-
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. - PubMed
-
- Fernandez A, Martins J, Villlafruela JJ, Marcen R, Pascual J, Cano T, et al. Variability of mycophenolate mofetil trough levels in stable kidney transplant patients. Transplant Proc. 2007;39:2185–2186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

