Angiotensin receptor type 1 antagonists protect against neuronal injury induced by oxygen-glucose depletion
- PMID: 20718738
- PMCID: PMC2962815
- DOI: 10.1111/j.1476-5381.2010.00840.x
Angiotensin receptor type 1 antagonists protect against neuronal injury induced by oxygen-glucose depletion
Abstract
Background and purpose: Several clinical trials and in vivo animal experiments have suggested that blockade of angiotensin receptor type 1 (AT(1)) improves ischaemic outcomes. However, the mechanism(s) underlying these effects has not been elucidated. Here, we have investigated the protective effects of pretreatment with AT(1) receptor antagonists, losartan or telmisartan, against ischaemic insult to neurons in vitro.
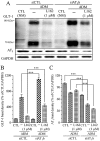
Experimental approach: Primary rat neuron-astrocyte co-cultures and astrocyte-defined medium (ADM)-cultured pure astrocyte cultures were prepared. Ischaemic injury was modelled by oxygen-glucose depletion (OGD) and lactate dehydrogenase release after OGD was measured with or without AT(1) receptor antagonists or agonists (L162313), AT(2) receptor antagonist (PD123319) or agonist (CGP-42112A) pretreatment, for 48 h. Activity of glutamate transporter 1 (GLT-1) was evaluated by [(3)H]-glutamate uptake assays, after AT(1) receptor agonists or antagonists. Immunoblot and real-time PCR were used for analysis of protein and mRNA levels of GLT-1.
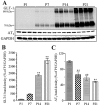
Key results: AT(1) receptor agonists augmented OGD-induced cellular damage, which was attenuated by AT(1) receptor antagonists. AT(1) receptor antagonists also suppressed OGD-induced extracellular glutamate release, reactive oxygen species production and nitric oxide generation. GLT-1 expression and glutamate uptake activity were significantly enhanced by AT(1) receptor antagonists and impaired by AT(1) receptor agonists. AT(1) receptor stimulation suppressed both ADM-induced GLT-1 protein expression and mRNA levels. AT(1)b receptor knock-down with siRNA enhanced GLT-1 expression. In postnatal (P1-P21) rat brains, protein levels of GLT-1 and AT(1) receptors were inversely correlated.
Conclusions and implications: Suppression of AT(1) receptor stimulation induced GLT-1 up-regulation, which ameliorated effects of ischaemic injury.
Figures







Similar articles
-
The mechanism of GLT-1 mediating cerebral ischemic injury depends on the activation of p38 MAPK.Brain Res Bull. 2019 Apr;147:1-13. doi: 10.1016/j.brainresbull.2019.01.028. Epub 2019 Feb 4. Brain Res Bull. 2019. PMID: 30731111
-
Mechanism Underlying Hyperbaric Oxygen's Effect on Nitric Oxide in an in Vitro Model of Traumatic Brain Injury.Discov Med. 2025 May;37(196):850-861. doi: 10.24976/Discov.Med.202537196.75. Discov Med. 2025. PMID: 40415360
-
Ischemic preconditioning reveals that GLT1/EAAT2 glutamate transporter is a novel PPARgamma target gene involved in neuroprotection.J Cereb Blood Flow Metab. 2007 Jul;27(7):1327-38. doi: 10.1038/sj.jcbfm.9600438. Epub 2007 Jan 10. J Cereb Blood Flow Metab. 2007. PMID: 17213861
-
Telmisartan ameliorates glutamate-induced neurotoxicity: roles of AT(1) receptor blockade and PPARγ activation.Neuropharmacology. 2014 Apr;79:249-61. doi: 10.1016/j.neuropharm.2013.11.022. Epub 2013 Dec 4. Neuropharmacology. 2014. PMID: 24316465 Free PMC article.
-
Carnosine decreased neuronal cell death through targeting glutamate system and astrocyte mitochondrial bioenergetics in cultured neuron/astrocyte exposed to OGD/recovery.Brain Res Bull. 2016 Jun;124:76-84. doi: 10.1016/j.brainresbull.2016.03.019. Epub 2016 Mar 31. Brain Res Bull. 2016. PMID: 27040711
Cited by
-
Potential of the angiotensin receptor blockers (ARBs) telmisartan, irbesartan, and candesartan for inhibiting the HMGB1/RAGE axis in prevention and acute treatment of stroke.Int J Mol Sci. 2013 Sep 13;14(9):18899-924. doi: 10.3390/ijms140918899. Int J Mol Sci. 2013. PMID: 24065095 Free PMC article. Review.
-
An integrative genome-wide transcriptome reveals that candesartan is neuroprotective and a candidate therapeutic for Alzheimer's disease.Alzheimers Res Ther. 2016 Jan 28;8:5. doi: 10.1186/s13195-015-0167-5. Alzheimers Res Ther. 2016. PMID: 26822027 Free PMC article.
-
The Possible Role of the Angiotensin System in the Pathophysiology of Schizophrenia: Implications for Pharmacotherapy.CNS Drugs. 2019 Jun;33(6):539-547. doi: 10.1007/s40263-019-00632-4. CNS Drugs. 2019. PMID: 30993607 Review.
-
Novel drugs and their targets in the potential treatment of diabetic retinopathy.Med Sci Monit. 2013 Apr 26;19:300-8. doi: 10.12659/MSM.883895. Med Sci Monit. 2013. PMID: 23619778 Free PMC article. Review.
-
Zanthoxylum bungeanum Maxim. (Rutaceae): A Systematic Review of Its Traditional Uses, Botany, Phytochemistry, Pharmacology, Pharmacokinetics, and Toxicology.Int J Mol Sci. 2017 Oct 18;18(10):2172. doi: 10.3390/ijms18102172. Int J Mol Sci. 2017. PMID: 29057808 Free PMC article.
References
-
- AbdAlla S, Lother H, el Missiry A, Langer A, Sergeev P, el Faramawy Y, et al. Angiotensin II AT2 receptor oligomers mediate G-protein dysfunction in an animal model of Alzheimer disease. J Biol Chem. 2009a;284:6554–6565. - PubMed
-
- AbdAlla S, Lother H, el Missiry A, Sergeev P, Langer A, el Faramawy Y, et al. Dominant negative AT2 receptor oligomers induce G-protein arrest and symptoms of neurodegeneration. J Biol Chem. 2009b;284:6566–6574. - PubMed
-
- Beal M. Mechanisms of excitotoxicity in neurologic diseases. FASEB J. 1992;6:3338–3344. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

