Valerenic acid derivatives as novel subunit-selective GABAA receptor ligands - in vitro and in vivo characterization
- PMID: 20718740
- PMCID: PMC2962817
- DOI: 10.1111/j.1476-5381.2010.00865.x
Valerenic acid derivatives as novel subunit-selective GABAA receptor ligands - in vitro and in vivo characterization
Abstract
Background and purpose: Subunit-specific modulators of gamma-aminobutyric acid (GABA) type A (GABA(A)) receptors can help to assess the physiological function of receptors with different subunit composition and also provide the basis for the development of new drugs. Valerenic acid (VA) was recently identified as a beta(2/3) subunit-specific modulator of GABA(A) receptors with anxiolytic potential. The aim of the present study was to generate VA derivatives as novel GABA(A) receptor modulators and to gain insight into the structure-activity relation of this molecule.
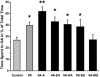
Experimental approach: The carboxyl group of VA was substituted by an uncharged amide or amides with different chain length. Modulation of GABA(A) receptors composed of different subunit compositions by the VA derivatives was studied in Xenopus oocytes by means of the two-microelectrode voltage-clamp technique. Half-maximal stimulation of GABA-induced chloride currents (I(GABA)) through GABA(A) receptors (EC(50)) and efficacies (maximal stimulation of I(GABA)) were estimated. Anxiolytic activity of the VA derivatives was studied in mice, applying the elevated plus maze test.
Key results: Valerenic acid amide (VA-A) displayed the highest efficacy (more than twofold greater I(GABA) enhancement than VA) and highest potency (EC(50)= 13.7 +/- 2.3 microM) on alpha(1)beta(3) receptors. Higher efficacy and potency of VA-A were also observed on alpha(1)beta(2)gamma(2s) and alpha(3)beta(3)gamma(2s) receptors. Anxiolytic effects were most pronounced for VA-A.
Conclusions and implications: Valerenic acid derivatives with higher efficacy and affinity can be generated. Greater in vitro action of the amide derivative correlated with a more pronounced anxiolytic effect in vivo. The data give further confidence in targeting beta(3) subunit containing GABA(A) receptors for development of anxiolytics.
Figures
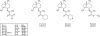

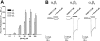
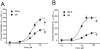
References
-
- Atack JR. The benzodiazepine binding site of GABA(A) receptors as a target for the development of novel anxiolytics. Expert Opin Investig Drugs. 2005;14:601–618. - PubMed
-
- Barnard EA, Skolnick P, Olsen RW, Möhler H, Sieghart W, Biggio G, et al. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. - PubMed
-
- Benke D, Barberis A, Kopp S, Altmann KH, Schubiger M, Vogt KE, et al. GABA A receptors as in vivo substrate for the anxiolytic action of valerenic acid, a major constituent of valerian root extracts. Neuropharmacology. 2009;56:174–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

