Phospholemman: a novel cardiac stress protein
- PMID: 20718822
- PMCID: PMC3013348
- DOI: 10.1111/j.1752-8062.2010.00213.x
Phospholemman: a novel cardiac stress protein
Abstract
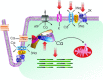
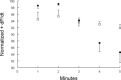
Phospholemman (PLM), a member of the FXYD family of regulators of ion transport, is a major sarcolemmal substrate for protein kinases A and C in cardiac and skeletal muscle. In the heart, PLM co-localizes and co-immunoprecipitates with Na(+)-K(+)-ATPase, Na(+)/Ca(2+) exchanger, and L-type Ca(2+) channel. Functionally, when phosphorylated at serine(68), PLM stimulates Na(+)-K(+)-ATPase but inhibits Na(+)/Ca(2+) exchanger in cardiac myocytes. In heterologous expression systems, PLM modulates the gating of cardiac L-type Ca(2+) channel. Therefore, PLM occupies a key modulatory role in intracellular Na(+) and Ca(2+) homeostasis and is intimately involved in regulation of excitation-contraction (EC) coupling. Genetic ablation of PLM results in a slight increase in baseline cardiac contractility and prolongation of action potential duration. When hearts are subjected to catecholamine stress, PLM minimizes the risks of arrhythmogenesis by reducing Na(+) overload and simultaneously preserves inotropy by inhibiting Na(+)/Ca(2+) exchanger. In heart failure, both expression and phosphorylation state of PLM are altered and may partly account for abnormalities in EC coupling. The unique role of PLM in regulation of Na(+)-K(+)-ATPase, Na(+)/Ca(2+) exchanger, and potentially L-type Ca(2+) channel in the heart, together with the changes in its expression and phosphorylation in heart failure, make PLM a rational and novel target for development of drugs in our armamentarium against heart failure. Clin Trans Sci 2010; Volume 3: 189-196.
Figures



Similar articles
-
Nitric oxide regulates cardiac intracellular Na⁺ and Ca²⁺ by modulating Na/K ATPase via PKCε and phospholemman-dependent mechanism.J Mol Cell Cardiol. 2013 Aug;61:164-71. doi: 10.1016/j.yjmcc.2013.04.013. Epub 2013 Apr 20. J Mol Cell Cardiol. 2013. PMID: 23612119 Free PMC article.
-
Coordinated regulation of cardiac Na(+)/Ca (2+) exchanger and Na (+)-K (+)-ATPase by phospholemman (FXYD1).Adv Exp Med Biol. 2013;961:175-90. doi: 10.1007/978-1-4614-4756-6_15. Adv Exp Med Biol. 2013. PMID: 23224879 Review.
-
Regulation of in vivo cardiac contractility by phospholemman: role of Na+/Ca2+ exchange.Am J Physiol Heart Circ Physiol. 2011 Mar;300(3):H859-68. doi: 10.1152/ajpheart.00894.2010. Epub 2010 Dec 30. Am J Physiol Heart Circ Physiol. 2011. PMID: 21193587 Free PMC article.
-
Expression and phosphorylation of the na-pump regulatory subunit phospholemman in heart failure.Circ Res. 2005 Sep 16;97(6):558-65. doi: 10.1161/01.RES.0000181172.27931.c3. Epub 2005 Aug 11. Circ Res. 2005. PMID: 16100047
-
Phospholemman: its role in normal cardiac physiology and potential as a druggable target in disease.Curr Opin Pharmacol. 2009 Apr;9(2):160-6. doi: 10.1016/j.coph.2008.12.015. Epub 2009 Feb 3. Curr Opin Pharmacol. 2009. PMID: 19195931 Review.
Cited by
-
Phospholemman deficiency in postinfarct hearts: enhanced contractility but increased mortality.Clin Transl Sci. 2012 Jun;5(3):235-42. doi: 10.1111/j.1752-8062.2012.00403.x. Epub 2012 Mar 27. Clin Transl Sci. 2012. PMID: 22686200 Free PMC article.
-
Late Sodium Current Inhibitors as Potential Antiarrhythmic Agents.Front Pharmacol. 2020 Apr 20;11:413. doi: 10.3389/fphar.2020.00413. eCollection 2020. Front Pharmacol. 2020. PMID: 32372952 Free PMC article. Review.
-
Nitric oxide regulates cardiac intracellular Na⁺ and Ca²⁺ by modulating Na/K ATPase via PKCε and phospholemman-dependent mechanism.J Mol Cell Cardiol. 2013 Aug;61:164-71. doi: 10.1016/j.yjmcc.2013.04.013. Epub 2013 Apr 20. J Mol Cell Cardiol. 2013. PMID: 23612119 Free PMC article.
-
Development of a high-affinity peptide that prevents phospholemman (PLM) inhibition of the sodium/calcium exchanger 1 (NCX1).Biochem J. 2016 Aug 1;473(15):2413-23. doi: 10.1042/BCJ20160465. Epub 2016 May 31. Biochem J. 2016. PMID: 27247424 Free PMC article.
-
Overexpression of the Na+/K+ ATPase α2 but not α1 isoform attenuates pathological cardiac hypertrophy and remodeling.Circ Res. 2014 Jan 17;114(2):249-256. doi: 10.1161/CIRCRESAHA.114.302293. Epub 2013 Nov 11. Circ Res. 2014. PMID: 24218169 Free PMC article.
References
-
- Presti CF, Jones LR, Lindemann JP. Isoproterenol‐induced phosphorylation of a 15‐kilodalton sarcolemmal protein in intact myocardium. J Biol Chem. 1985; 260: 3860–3867. - PubMed
-
- Presti CF, Scott BT, Jones LR. Identification of an endogenous protein kinase C activity and its intrinsic 15‐kilodalton substrate in purified canine cardiac sarcolemmal vesicles. J Biol Chem. 1985; 260: 13879–13889. - PubMed
-
- Lindemann JP. α‐adrenergic stimulation of sarcolemmal protein phosphorylation and slow responses in intact myocardium. J Biol Chem. 1986; 261: 4860–4867. - PubMed
-
- Palmer CJ, Scott BT, Jones LR. Purification and complete sequence determination of the major plasma membrane substrate for cAMP‐dependent protein kinase and protein kinase C in myocardium. J Biol Chem. 1991; 266: 11126–11130. - PubMed
-
- Chen LS, Lo CF, Numann R, Cuddy M. Characterization of the human and rat phospholemman (PLM) cDNAs and localization of the human PLM gene to chromosome 19q13.1. Genomics. 1997; 41: 435–443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

